Abstract
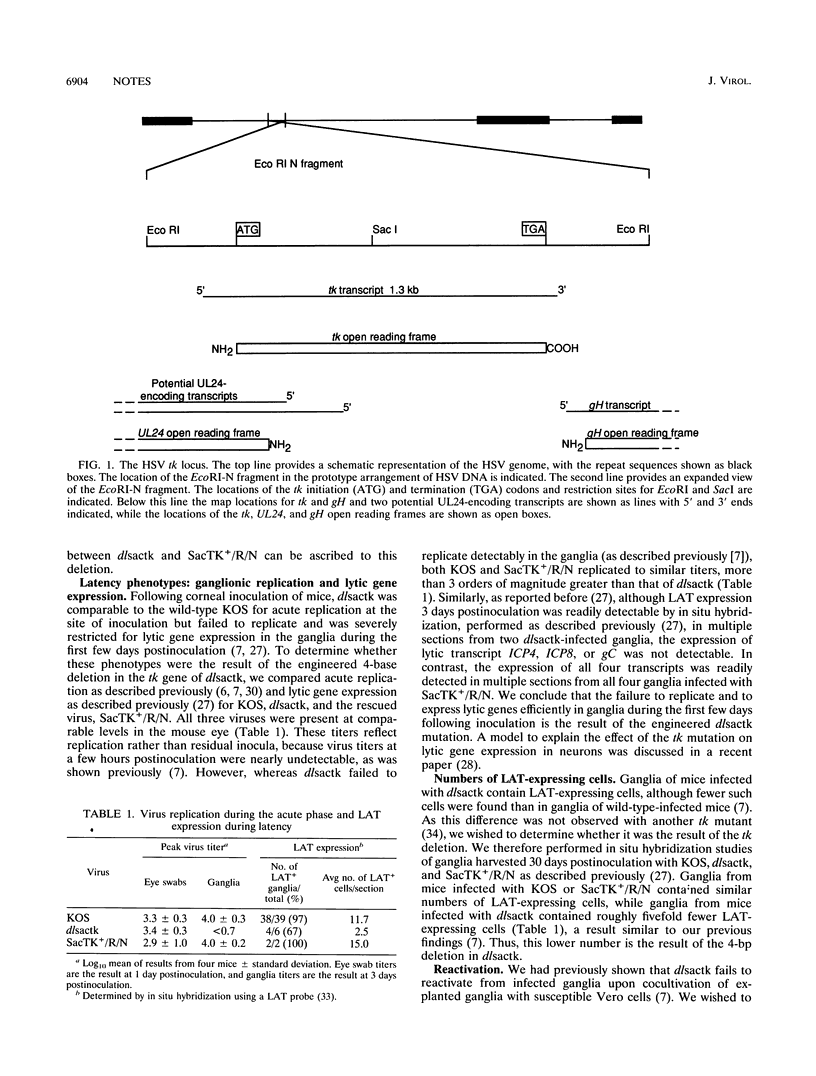
From marker rescue, sequencing, transcript, and latency analyses of the thymidine kinase-negative herpes simplex virus mutant dlsactk and studies using the thymidine kinase inhibitor Ro 31-5140, we infer that the virus-encoded thymidine kinase is required in murine trigeminal ganglia for acute replication and lytic gene expression, for increasing the numbers of cells expressing latency-associated transcripts, and for reactivation from latent infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne N., Bravo F. J., Ashton W. T., Meurer L. C., Tolman R. L., Karkas J. D., Stanberry L. R. Assessment of a selective inhibitor of herpes simplex virus thymidine kinase (L-653,180) as therapy for experimental recurrent genital herpes. Antimicrob Agents Chemother. 1992 Sep;36(9):2020–2024. doi: 10.1128/aac.36.9.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione-Piccardo J., Rawls W. E., Bacchetti S. Selective assay for herpes simplex viruses expressing thymidine kinase. J Virol. 1979 Aug;31(2):281–287. doi: 10.1128/jvi.31.2.281-287.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Crumpacker C. S. Resistance of herpesviruses to antiviral drugs. Antimicrob Agents Chemother. 1992 Aug;36(8):1589–1595. doi: 10.1128/aac.36.8.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Weinheimer S. P., McKnight S. L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986 Oct 3;234(4772):53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Kemp S., Darby G., Minson A. C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989 Apr;70(Pt 4):869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Papavassiliou A. G., Rice M., Hecht L. B., Silverstein S., Wagner E. K. Analysis of the herpes simplex virus type 1 promoter controlling the expression of UL38, a true late gene involved in capsid assembly. J Virol. 1991 Feb;65(2):769–786. doi: 10.1128/jvi.65.2.769-786.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Weisshart K., Digard P., deBruynKops A., Knipe D. M., Coen D. M. Polymerization activity of an alpha-like DNA polymerase requires a conserved 3'-5' exonuclease active site. Mol Cell Biol. 1991 Sep;11(9):4786–4795. doi: 10.1128/mcb.11.9.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y., Gilden D. H., Shtram Y., Asher Y., Tabor E., Wellish M., Devlin M., Snipper D., Hadar J., Becker Y. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76(1):39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988 Nov;167(1):279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J. G., Martin S. L., Coen D. M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989 Apr;63(4):1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Everett R. D. The control of herpes simplex virus type-1 late gene transcription: a 'TATA-box'/cap site region is sufficient for fully efficient regulated activity. Nucleic Acids Res. 1986 Nov 11;14(21):8247–8264. doi: 10.1093/nar/14.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. P., Bodin E. T., Coen D. M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990 Sep;64(9):4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman H. E., Varnell E. D., Cheng Y. C., Bobek M., Thompson H. W., Dutschman G. E. Suppression of ocular herpes recurrences by a thymidine kinase inhibitor in squirrel monkeys. Antiviral Res. 1991 Oct;16(3):227–232. doi: 10.1016/0166-3542(91)90002-9. [DOI] [PubMed] [Google Scholar]
- Kibler P. K., Duncan J., Keith B. D., Hupel T., Smiley J. R. Regulation of herpes simplex virus true late gene expression: sequences downstream from the US11 TATA box inhibit expression from an unreplicated template. J Virol. 1991 Dec;65(12):6749–6760. doi: 10.1128/jvi.65.12.6749-6760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Czelusniak S. M. Effect of a thymidine kinase inhibitor (L-653,180) on antiviral treatment of experimental herpes simplex virus infection in mice. Antiviral Res. 1990 Oct-Nov;14(4-5):207–214. doi: 10.1016/0166-3542(90)90002-o. [DOI] [PubMed] [Google Scholar]
- Kosz-Vnenchak M., Coen D. M., Knipe D. M. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J Virol. 1990 Nov;64(11):5396–5402. doi: 10.1128/jvi.64.11.5396-5402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosz-Vnenchak M., Jacobson J., Coen D. M., Knipe D. M. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J Virol. 1993 Sep;67(9):5383–5393. doi: 10.1128/jvi.67.9.5383-5393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Nadeau K. C., Rundle S. A., Schaffer P. A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Ruffner K. L., Hildebrand C., Schaffer P. A., Wright G. E., Coen D. M. Specific inhibitors of herpes simplex virus thymidine kinase diminish reactivation of latent virus from explanted murine ganglia. Antimicrob Agents Chemother. 1990 Jun;34(6):1285–1286. doi: 10.1128/aac.34.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Sandri-Goldin R. M., Stevens J. G. Latent infections in spinal ganglia with thymidine kinase-deficient herpes simplex virus. J Virol. 1989 Nov;63(11):4976–4978. doi: 10.1128/jvi.63.11.4976-4978.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Smiley J. R., Leslie P., Brais J., Rudzroga H. E., Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984 Sep;51(3):747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Mavromara-Nazos P., Sears A. E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988 Jan;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988 Sep;158(3):602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Meignier B., Martin B., Whitley R. J., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J Infect Dis. 1990 Aug;162(2):313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- Nsiah Y. A., Tolman R. L., Karkas J. D., Rapp F. Suppression of herpes simplex virus type 1 reactivation from latency by (+-)-9-([(Z)-2-(hydroxymethyl)cyclohexyl]methyl) guanine (L-653,180) in vitro. Antimicrob Agents Chemother. 1990 Aug;34(8):1551–1555. doi: 10.1128/aac.34.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Price R. W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect Immun. 1981 Nov;34(2):571–580. doi: 10.1128/iai.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Sharp J. A., Summers W. C. In vitro and in vivo transcription initiation sites on the TK-encoding BamHI Q fragment of HSV-1 DNA. Virology. 1984 Oct 30;138(2):368–372. doi: 10.1016/0042-6822(84)90363-5. [DOI] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., Meignier B., Roizman B. Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol. 1985 Aug;55(2):410–416. doi: 10.1128/jvi.55.2.410-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffy K. R., Weir J. P. Upstream promoter elements of the herpes simplex virus type 1 glycoprotein H gene. J Virol. 1991 Feb;65(2):972–975. doi: 10.1128/jvi.65.2.972-975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A. Thymidine kinase (TK) activity in herpes simplex virus type 1 recombinants that carry insertions affecting regulation of the TK gene. Virology. 1986 Nov;155(1):257–261. doi: 10.1016/0042-6822(86)90185-6. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus after in vivo complementation. J Virol. 1987 Jul;61(7):2171–2174. doi: 10.1128/jvi.61.7.2171-2174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hay K. A., Edris W. A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989 Jun;63(6):2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Tenser R. B. Role of herpes simplex virus thymidine kinase expression in viral pathogenesis and latency. Intervirology. 1991;32(2):76–92. doi: 10.1159/000150188. [DOI] [PubMed] [Google Scholar]
- Wilcox C. L., Crnic L. S., Pizer L. I. Replication, latent infection, and reactivation in neuronal culture with a herpes simplex virus thymidine kinase-negative mutant. Virology. 1992 Mar;187(1):348–352. doi: 10.1016/0042-6822(92)90326-k. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Eglin R. P., Sanders P. G., Clements J. B. The association of herpes simplex virus with squamous carcinoma of the cervix, and studies of the virus thymidine kinase gene. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):411–421. doi: 10.1098/rspb.1980.0143. [DOI] [PubMed] [Google Scholar]
- Wobbe K. K., Digard P., Staknis D., Coen D. M. Unusual regulation of expression of the herpes simplex virus DNA polymerase gene. J Virol. 1993 Sep;67(9):5419–5425. doi: 10.1128/jvi.67.9.5419-5425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]