Abstract
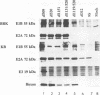
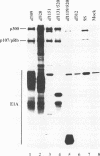
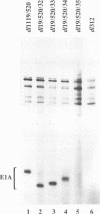
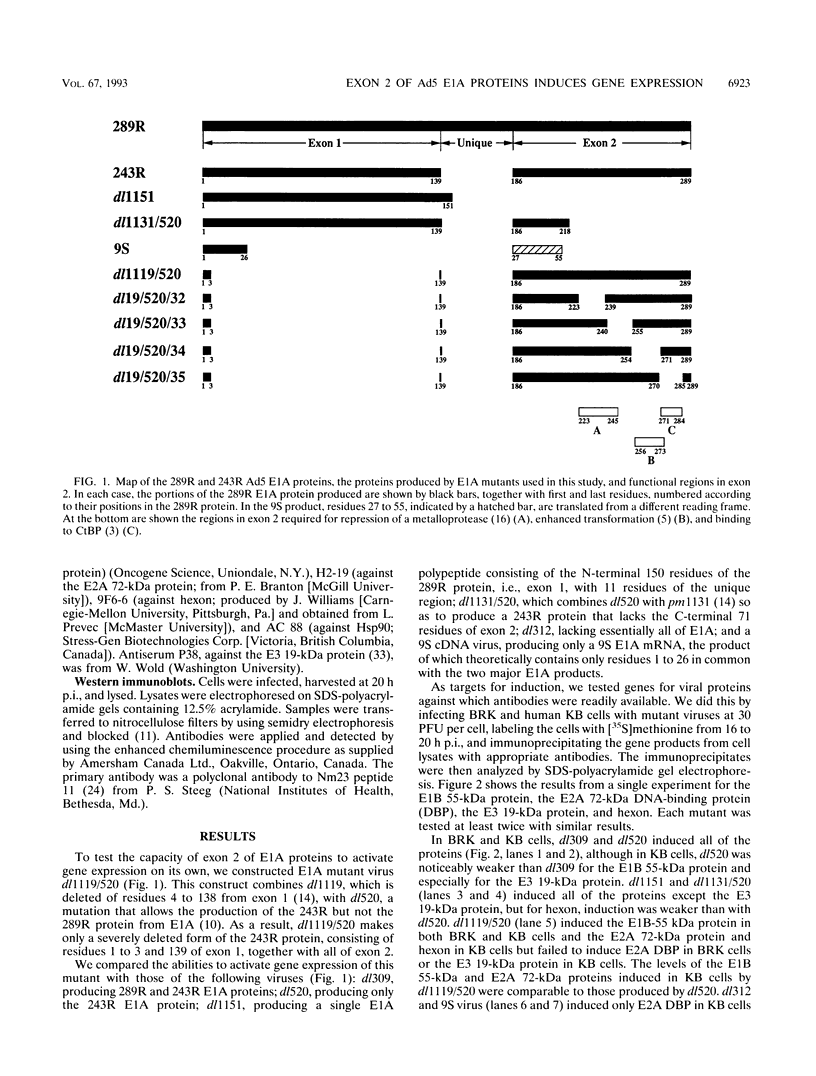
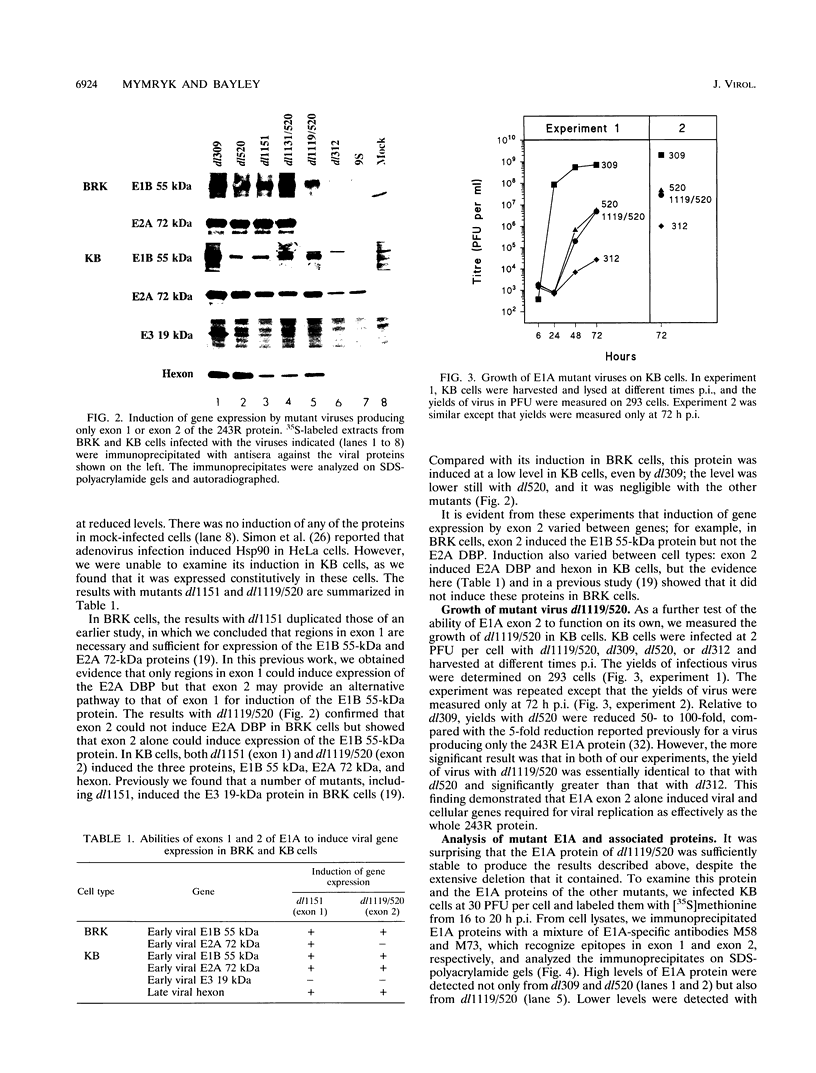
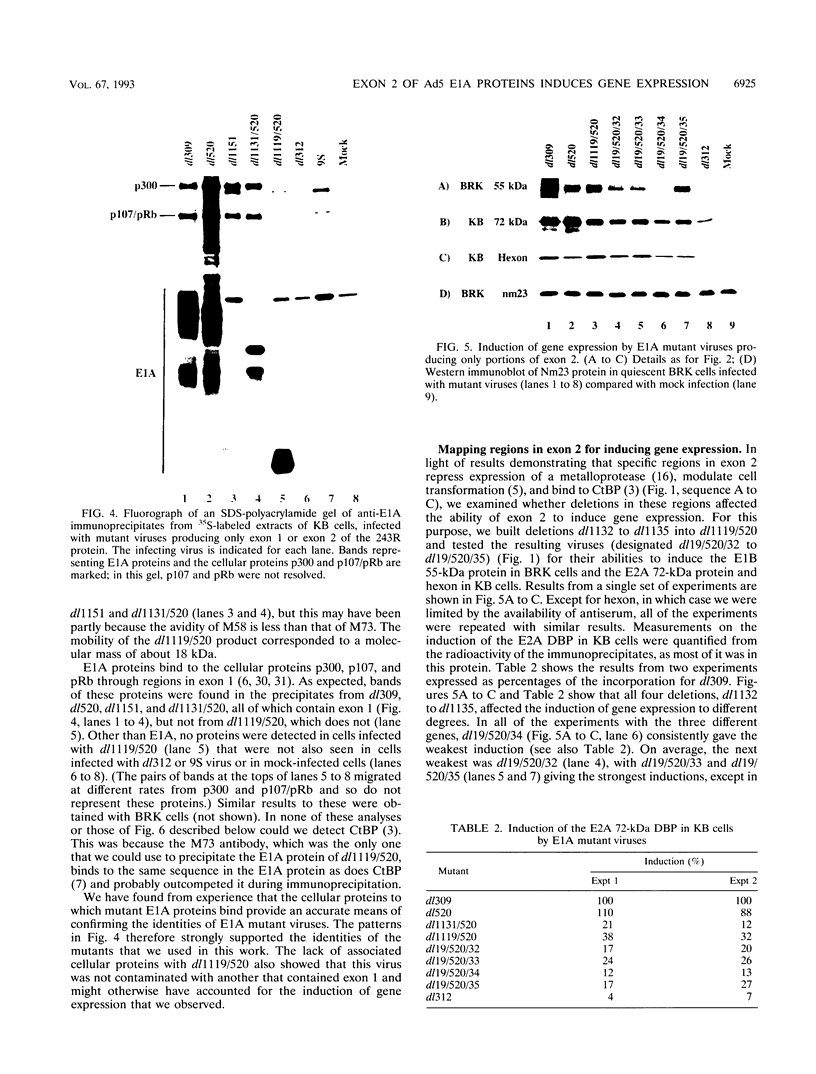
We have constructed an adenovirus type 5 (Ad5) E1A mutant, dl1119/520, that produces essentially only exon 2 of the major E1A proteins. In infected primary baby rat kidney cells, this mutant induced expression of the E1B 55-kDa protein, and in infected human KB cells, it induced expression of this protein, the E2A 72-kDa protein, and hexon. In KB cells, this mutant grew substantially better than Ad5 dl312, which lacks E1A, and as well as Ad5 dl520, an E1A mutant producing only the 243-residue protein. These results suggest that exon 2 of E1A proteins on its own was able to activate gene expression. We also constructed mutants of dl1119/520, containing small deletions in regions of exon 2 that others found to be associated with effects on the properties of E1A transformants. None of these deletions destroyed gene activation completely, indicating that there may be some redundancy among sequences in exon 2 for inducing gene expression. The two deletions that decreased induction the most, residues 224 to 238 and 255 to 270, were in regions reported to be associated with the expression of a metalloprotease and with enhanced transformation, suggesting that exon 2 may regulate expression of genes governing cell growth. It is remarkable that all sections of E1A proteins, exon 1, the unique region, and exon 2, have now been found to affect gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellgrau D., Walker T. A., Cook J. L. Recognition of adenovirus E1A gene products on immortalized cell surfaces by cytotoxic T lymphocytes. J Virol. 1988 May;62(5):1513–1519. doi: 10.1128/jvi.62.5.1513-1519.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesson M., Svensson C., Linder S., Akusjärvi G. The carboxy-terminal exon of the adenovirus E1A protein is required for E4F-dependent transcription activation. EMBO J. 1992 Sep;11(9):3347–3354. doi: 10.1002/j.1460-2075.1992.tb05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. M., Subramanian T., Schaeper U., La Regina M., Bayley S., Chinnadurai G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993 Feb;12(2):469–478. doi: 10.1002/j.1460-2075.1993.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-González R., Glenney J. R., Jr Temperature-dependent tyrosine phosphorylation of microtubule-associated protein kinase in epidermal growth factor-stimulated human fibroblasts. Cell Regul. 1991 Aug;2(8):663–673. doi: 10.1091/mbc.2.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. L., Gopalakrishnan S., Quinlan M. P. Modulation of transformation of primary epithelial cells by the second exon of the Ad5 E1A12S gene. Oncogene. 1991 Nov;6(11):2093–2103. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedrich R. W., Bayley S. T., Engel D. A. Induction of AP-1 DNA-binding activity and c-fos mRNA by the adenovirus 243R E1A protein and cyclic AMP requires domains necessary for transformation. J Virol. 1992 Oct;66(10):5849–5859. doi: 10.1128/jvi.66.10.5849-5859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Bayley S. T. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology. 1992 Jan;186(1):15–24. doi: 10.1016/0042-6822(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Evelegh C. M., Cunniff N. F., Skiadopoulos M. H., Floroff M. R., Denman J. E., Bayley S. T. Use of deletion and point mutants spanning the coding region of the adenovirus 5 E1A gene to define a domain that is essential for transcriptional activation. Virology. 1988 Apr;163(2):494–502. doi: 10.1016/0042-6822(88)90290-5. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S., Popowicz P., Svensson C., Marshall H., Bondesson M., Akusjärvi G. Enhanced invasive properties of rat embryo fibroblasts transformed by adenovirus E1A mutants with deletions in the carboxy-terminal exon. Oncogene. 1992 Mar;7(3):439–443. [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Moran E., Grodzicker T., Roberts R. J., Mathews M. B., Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. doi: 10.1128/jvi.57.3.765-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymryk J. S., Lee R. W., Bayley S. T. Ability of adenovirus 5 E1A proteins to suppress differentiation of BC3H1 myoblasts correlates with their binding to a 300 kDa cellular protein. Mol Biol Cell. 1992 Oct;3(10):1107–1115. doi: 10.1091/mbc.3.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Douglas J. L. Immortalization of primary epithelial cells requires first- and second-exon functions of adenovirus type 5 12S. J Virol. 1992 Apr;66(4):2020–2030. doi: 10.1128/jvi.66.4.2020-2030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Whyte P., Grodzicker T. Growth factor induction by the adenovirus type 5 E1A 12S protein is required for immortalization of primary epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3191–3203. doi: 10.1128/mcb.8.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengard A. M., Krutzsch H. C., Shearn A., Biggs J. R., Barker E., Margulies I. M., King C. R., Liotta L. A., Steeg P. S. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989 Nov 9;342(6246):177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Kitchener K., Kao H. T., Hickey E., Weber L., Voellmy R., Heintz N., Nevins J. R. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. doi: 10.1128/mcb.7.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Pozzatti R., Liotta L. A., Sobel M. E. Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res. 1988 Nov 15;48(22):6550–6554. [PubMed] [Google Scholar]
- Subramanian T., La Regina M., Chinnadurai G. Enhanced ras oncogene mediated cell transformation and tumorigenesis by adenovirus 2 mutants lacking the C-terminal region of E1a protein. Oncogene. 1989 Apr;4(4):415–420. [PubMed] [Google Scholar]
- Urbanelli D., Sawada Y., Raskova J., Jones N. C., Shenk T., Raska K., Jr C-terminal domain of the adenovirus E1A oncogene product is required for induction of cytotoxic T lymphocytes and tumor-specific transplantation immunity. Virology. 1989 Dec;173(2):607–614. doi: 10.1016/0042-6822(89)90572-2. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Deutscher S. L., Kapoor Q. S. The 19-kDa glycoprotein coded by region E3 of adenovirus. Purification, characterization, and structural analysis. J Biol Chem. 1985 Feb 25;260(4):2424–2431. [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]