Abstract
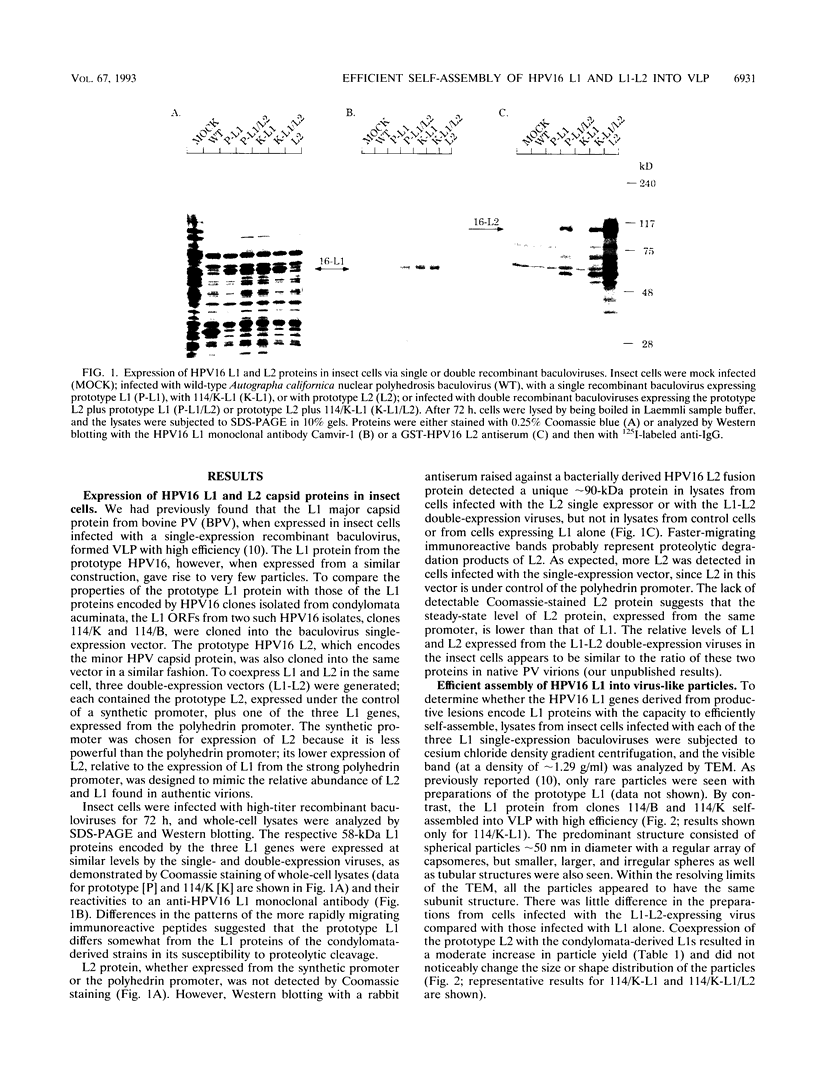
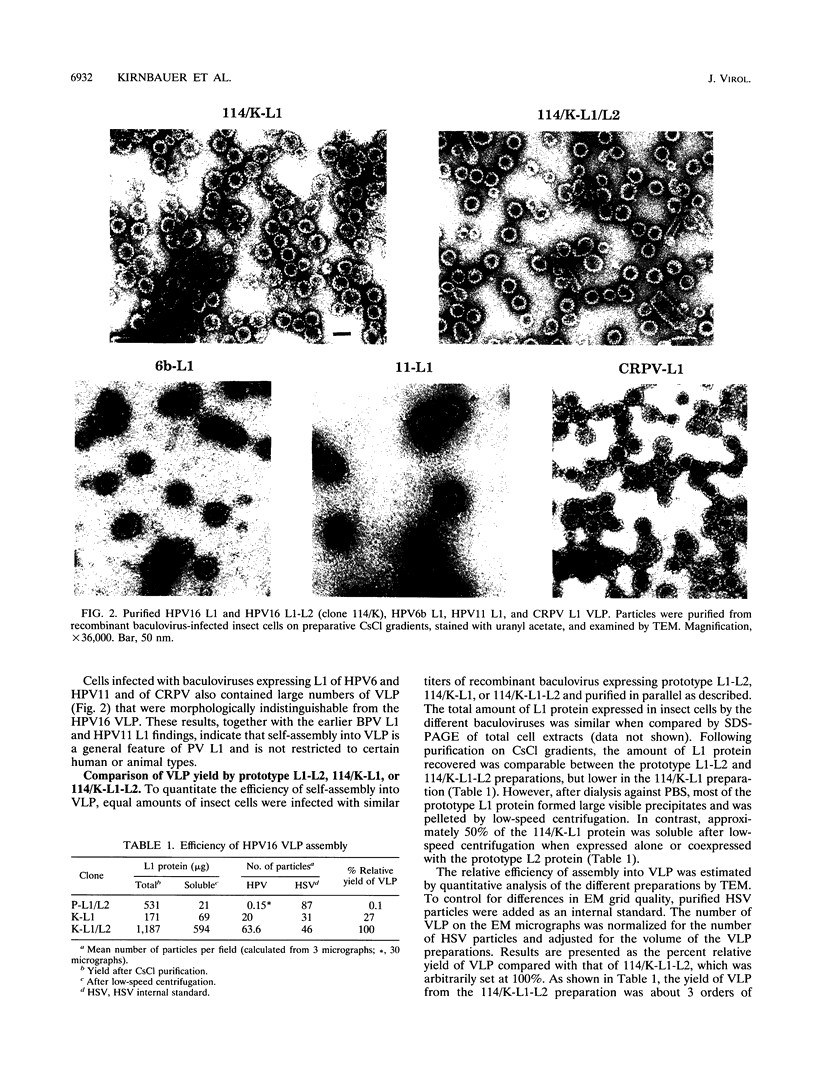
The L1 genes of two human papillomavirus type 16 (HPV16) isolates derived from condylomata acuminata were used to express the L1 major capsid protein in insect cells via recombinant baculoviruses. Both L1 major capsid proteins self-assembled into virus-like particles (VLP) with high efficiency and could be purified in preparative amounts on density gradients. The yield of VLP was 3 orders of magnitude higher than what has been obtained previously, using L1 derived from the prototype HPV16. DNA sequence comparison identified a single nonconserved amino acid change to be responsible for the inefficient self-assembly of the prototype L1. VLP were also obtained by expressing L1 of HPV6, HPV11, and cottontail rabbit papillomavirus, indicating that L1 from a variety of papillomaviruses has the intrinsic capacity to self-assemble into VLP. Coexpression of HPV16 L1 plus L2 by using a baculovirus double-expression vector also resulted in efficient self-assembly of VLP, and the average particle yield increased about fourfold in comparison to when L1 only was expressed. Coimmunoprecipitation of L1 and L2 and cosedimentation of the two proteins in a sucrose gradient demonstrated that L2 was incorporated into the particles. The ability to generate preparative amounts of HPV16 L1 and L1-L2 VLP may have implications for the development of a serological assay to detect anti-HPV16 virion immune responses to conformational epitopes and for immunoprophylaxis against HPV16 infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen N. D., Kreider J. W., Cladel N. M., Patrick S. D., Welsh P. A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990 Nov;64(11):5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollard S. C., Wilson J. L., Demeter L. M., Bonnez W., Reichman R. C., Broker T. R., Chow L. T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992 Jul;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- Galloway D. A. Serological assays for the detection of HPV antibodies. IARC Sci Publ. 1992;(119):147–161. [PubMed] [Google Scholar]
- Ghim S., Christensen N. D., Kreider J. W., Jenson A. B. Comparison of neutralization of BPV-1 infection of C127 cells and bovine fetal skin xenografts. Int J Cancer. 1991 Sep 9;49(2):285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Diehl V., Schultz-Coulon H. J., zur Hausen H. Molecular cloning and characterization of human papilloma virus DNA derived from a laryngeal papilloma. J Virol. 1982 Oct;44(1):393–400. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee M. E., Yaegashi N., Galloway D. A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993 Jan;67(1):315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C. L., Galloway D. A. Identification of the E5 open reading frame of human papillomavirus type 16. J Virol. 1988 Mar;62(3):1071–1075. doi: 10.1128/jvi.62.3.1071-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett W. F., Smith K. T., O'Neil B. W., Gaukroger J. M., Chandrachud L. M., Grindlay G. J., McGarvie G. M., Campo M. S. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991 Sep;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D. R., Schiller J. T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992 Apr;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Meyers C., Frattini M. G., Hudson J. B., Laimins L. A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Meyers C., Wettstein F. O. Genetic analysis of CRPV pathogenesis: the L1 open reading frame is dispensable for cellular transformation but is required for papilloma formation. Virology. 1989 May;170(1):321–325. doi: 10.1016/0042-6822(89)90388-7. [DOI] [PubMed] [Google Scholar]
- Parton A. Nucleotide sequence of the HPV16 L1 open reading frame. Nucleic Acids Res. 1990 Jun 25;18(12):3631–3631. doi: 10.1093/nar/18.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C., Bonnez W., Reichman R. C., Garcea R. L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993 Apr;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M. H. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 1992 Mar 18;84(6):394–398. doi: 10.1093/jnci/84.6.394. [DOI] [PubMed] [Google Scholar]
- Sterling J., Stanley M., Gatward G., Minson T. Production of human papillomavirus type 16 virions in a keratinocyte cell line. J Virol. 1990 Dec;64(12):6305–6307. doi: 10.1128/jvi.64.12.6305-6307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Z., Ooi B. G., Miller L. K. Baculovirus vectors for multiple gene expression and for occluded virus production. Gene. 1991 Apr;100:131–137. doi: 10.1016/0378-1119(91)90358-i. [DOI] [PubMed] [Google Scholar]
- Xi S. Z., Banks L. M. Baculovirus expression of the human papillomavirus type 16 capsid proteins: detection of L1-L2 protein complexes. J Gen Virol. 1991 Dec;72(Pt 12):2981–2988. doi: 10.1099/0022-1317-72-12-2981. [DOI] [PubMed] [Google Scholar]
- Zhou J., Sun X. Y., Stenzel D. J., Frazer I. H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991 Nov;185(1):251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M., Gissmann L., zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981 Dec;40(3):932–935. doi: 10.1128/jvi.40.3.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Viruses in human cancers. Science. 1991 Nov 22;254(5035):1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]






