Abstract
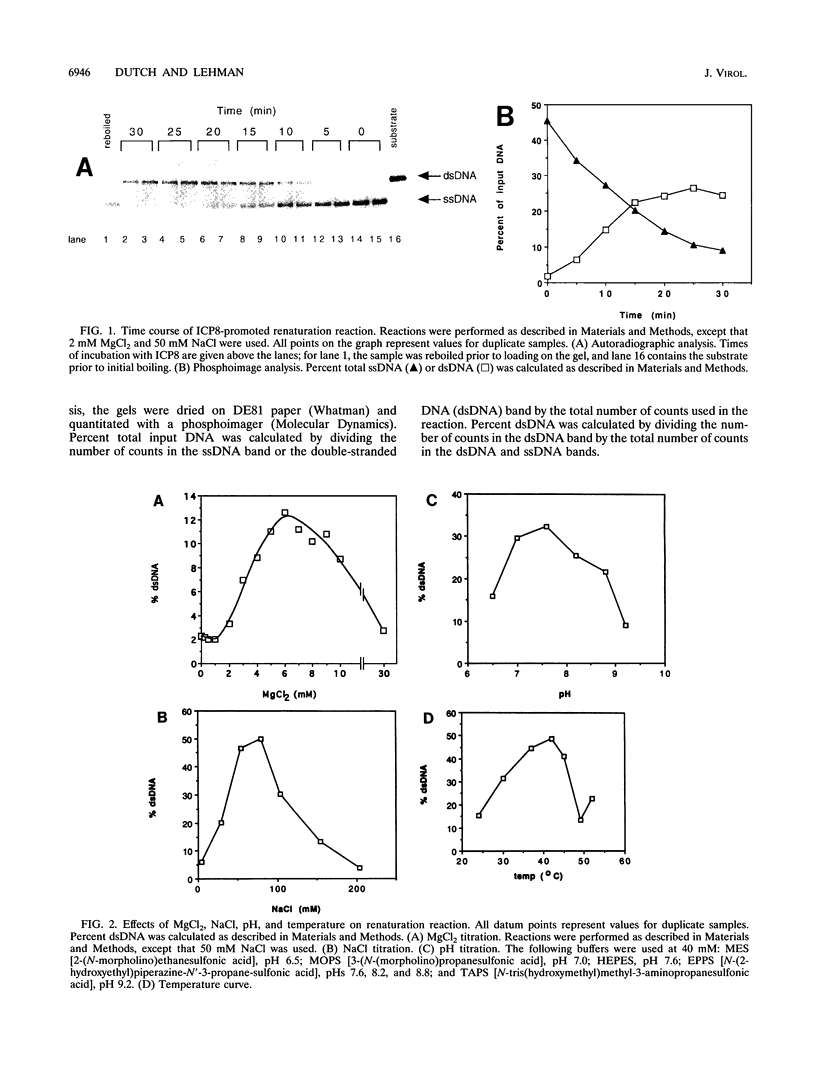
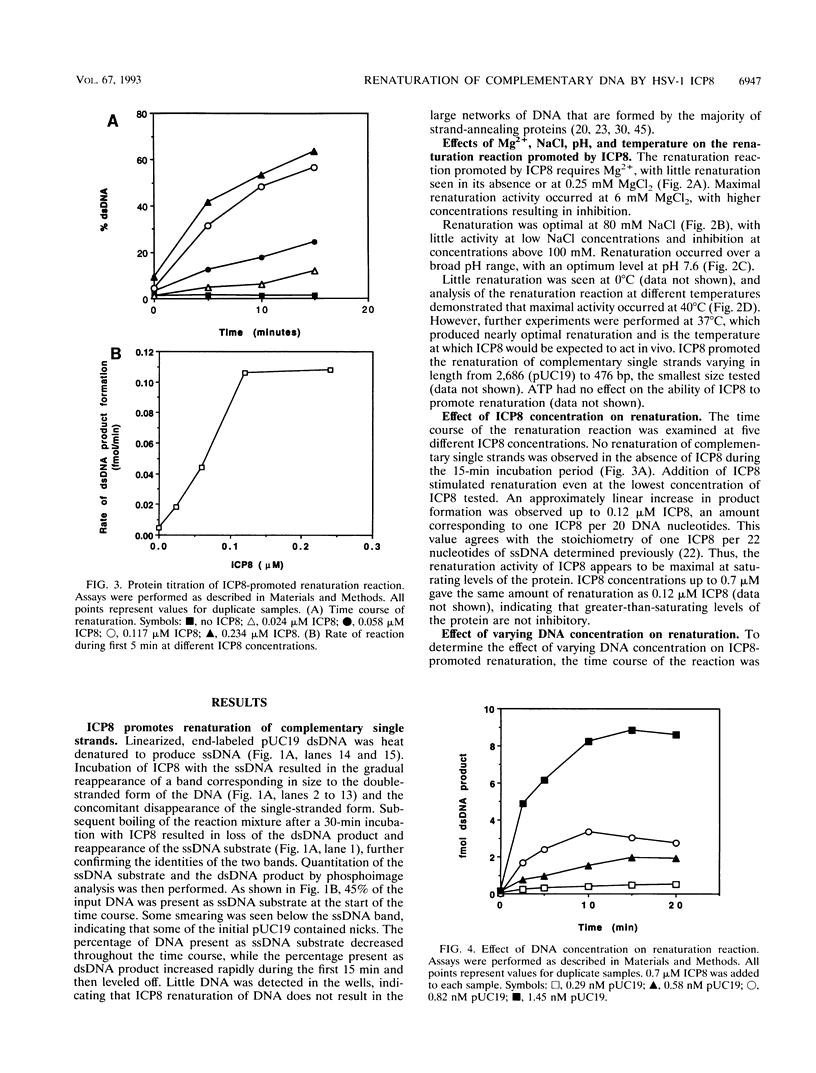
ICP8, the major single-stranded DNA-binding protein of herpes simplex virus type 1, promotes renaturation of complementary single strands of DNA. This reaction is ATP independent but requires Mg2+. The activity is maximal at pH 7.6 and 80 mM NaCl. The major product of the reaction is double-stranded DNA, and no evidence of large DNA networks is seen. The reaction occurs at subsaturating concentrations of ICP8 but reaches maximal levels with saturating concentrations of ICP8. Finally, the renaturation reaction is second order with respect to DNA concentration. The ability of ICP8 to promote the renaturation of complementary single strands suggests a role for ICP8 in the high level of recombination seen in cells infected with herpes simplex virus type 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Boehmer P. E., Dodson M. S., Lehman I. R. The herpes simplex virus type-1 origin binding protein. DNA helicase activity. J Biol Chem. 1993 Jan 15;268(2):1220–1225. [PubMed] [Google Scholar]
- Boehmer P. E., Lehman I. R. Herpes simplex virus type 1 ICP8: helix-destabilizing properties. J Virol. 1993 Feb;67(2):711–715. doi: 10.1128/jvi.67.2.711-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner C., Hernandez T. R., Lehman I. R., Griffith J. Herpes simplex virus 1 single-strand DNA-binding protein (ICP8) will promote homologous pairing and strand transfer. J Mol Biol. 1993 May 20;231(2):241–250. doi: 10.1006/jmbi.1993.1279. [DOI] [PubMed] [Google Scholar]
- Bryant F. R., Lehman I. R. On the mechanism of renaturation of complementary DNA strands by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jan;82(2):297–301. doi: 10.1073/pnas.82.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush M., Yager D. R., Gao M., Weisshart K., Marcy A. I., Coen D. M., Knipe D. M. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J Virol. 1991 Mar;65(3):1082–1089. doi: 10.1128/jvi.65.3.1082-1089.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou H. C., Weller S. K., Coen D. M. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985 Sep;145(2):213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Baldwin R. L. Catalysis of DNA reassociation by the Escherichia coli DNA binding protein: A polyamine-dependent reaction. J Mol Biol. 1977 Sep 25;115(3):441–454. doi: 10.1016/0022-2836(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Crute J. J., Lehman I. R. Herpes simplex virus-1 helicase-primase. Physical and catalytic properties. J Biol Chem. 1991 Mar 5;266(7):4484–4488. [PubMed] [Google Scholar]
- Delius H., Clements J. B. A partial denaturation map of herpes simplex virus type 1 DNA: evidence for inversions of the unique DNA regions. J Gen Virol. 1976 Oct;33(1):125–133. doi: 10.1099/0022-1317-33-1-125. [DOI] [PubMed] [Google Scholar]
- Dodson M. S., Lehman I. R. The herpes simplex virus type I origin binding protein. DNA-dependent nucleoside triphosphatase activity. J Biol Chem. 1993 Jan 15;268(2):1213–1219. [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J Virol. 1991 May;65(5):2666–2675. doi: 10.1128/jvi.65.5.2666-2675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J Virol. 1983 Sep;47(3):478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte S. S., Olson J. W., Ruyechan W. T. The major herpes simplex virus type-1 DNA-binding protein is a zinc metalloprotein. J Biol Chem. 1991 Jun 25;266(18):11413–11416. [PubMed] [Google Scholar]
- Hall S. D., Kane M. F., Kolodner R. D. Identification and characterization of the Escherichia coli RecT protein, a protein encoded by the recE region that promotes renaturation of homologous single-stranded DNA. J Bacteriol. 1993 Jan;175(1):277–287. doi: 10.1128/jb.175.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez T. R., Lehman I. R. Functional interaction between the herpes simplex-1 DNA polymerase and UL42 protein. J Biol Chem. 1990 Jul 5;265(19):11227–11232. [PubMed] [Google Scholar]
- Heyer W. D., Evans D. H., Kolodner R. D. Renaturation of DNA by a Saccharomyces cerevisiae protein that catalyzes homologous pairing and strand exchange. J Biol Chem. 1988 Oct 15;263(29):15189–15195. [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Beta protein of bacteriophage lambda promotes renaturation of DNA. J Biol Chem. 1981 Dec 25;256(24):12636–12639. [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. An immunoassay for the study of DNA-binding activities of herpes simplex virus protein ICP8. J Virol. 1985 Jun;54(3):731–738. doi: 10.1128/jvi.54.3.731-738.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Purifoy D., Minson A., Powell K. L. Herpes simplex virus non-structural proteins. III. Function of the major DNA-binding protein. J Gen Virol. 1983 May;64(Pt 5):983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- McEntee K. Kinetics of DNA renaturation catalyzed by the RecA protein of Escherichia coli. Biochemistry. 1985 Jul 30;24(16):4345–4351. doi: 10.1021/bi00337a014. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Site-specific inversion sequence of the herpes simplex virus genome: domain and structural features. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7047–7051. doi: 10.1073/pnas.78.11.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa K., Radding C. M. The homologous recombination system of phage lambda. Pairing activities of beta protein. J Biol Chem. 1986 Jun 5;261(16):7472–7478. [PubMed] [Google Scholar]
- Norris D., Kolodner R. Interaction of a Saccharomyces cerevisiae strand exchange stimulatory factor with DNA. Biochemistry. 1990 Aug 28;29(34):7911–7917. doi: 10.1021/bi00486a019. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E., Elias P., Funnell B. E., Lehman I. R. Interaction between the DNA polymerase and single-stranded DNA-binding protein (infected cell protein 8) of herpes simplex virus 1. J Biol Chem. 1987 Mar 25;262(9):4260–4266. [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J Virol. 1989 Jan;63(1):196–204. doi: 10.1128/jvi.63.1.196-204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius B. W., Berg P. Renaturation of complementary DNA strands mediated by purified mammalian heterogeneous nuclear ribonucleoprotein A1 protein: implications for a mechanism for rapid molecular assembly. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8403–8407. doi: 10.1073/pnas.87.21.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T. The major herpes simplex virus DNA-binding protein holds single-stranded DNA in an extended configuration. J Virol. 1983 May;46(2):661–666. doi: 10.1128/jvi.46.2.661-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Weir A. C. Interaction with nucleic acids and stimulation of the viral DNA polymerase by the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1984 Dec;52(3):727–733. doi: 10.1128/jvi.52.3.727-733.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D. Herpes simplex virus type 1 origin-dependent DNA replication in insect cells using recombinant baculoviruses. J Gen Virol. 1992 Feb;73(Pt 2):313–321. doi: 10.1099/0022-1317-73-2-313. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Gao M., Knipe D. M., Powell K. L. Association between the herpes simplex virus major DNA-binding protein and alkaline nuclease. J Virol. 1992 Feb;66(2):1152–1161. doi: 10.1128/jvi.66.2.1152-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Vaughan P. J., Purifoy D. J., Powell K. L. DNA-binding protein associated with herpes simplex virus DNA polymerase. J Virol. 1985 Feb;53(2):501–508. doi: 10.1128/jvi.53.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. S., Hall J. D. Characterization of a major DNA-binding domain in the herpes simplex virus type 1 DNA-binding protein (ICP8). J Virol. 1990 May;64(5):2082–2089. doi: 10.1128/jvi.64.5.2082-2089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]