Abstract
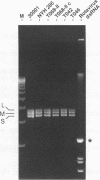
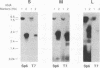
Previous studies demonstrated that some isolates of the sexually transmitted protozoan Trichomonas vaginalis are infected with a nonsegmented, double-stranded RNA (dsRNA) virus. A reexamination of the total dsRNA extracted from several virus-harboring isolates indicated the presence of at least three dsRNAs with sizes ranging from 4.8 to 4.3 kbp. The double-stranded nature of each of the three segments was determined by hybridization experiments using riboprobes of opposite polarities obtained from cDNA generated to each of the segments. All three segments were present in agar clones originating from single organisms of T. vaginalis isolates, suggesting that the three segments were not the result of a mixed population of trichomonads harboring different sizes of dsRNA. The three segments were associated with CsCl-purified virus particles, as evidenced by electron microscopy, and RNAse treatment of the preparation containing virus particles did not destroy the dsRNAs. Finally, the individual dsRNA segments were purified for use as probes to determine whether the three dsRNAs shared any sequence homology. Each end-labeled dsRNA segment did not cross-hybridize to any of the other two segments, a finding consistent with the hybridization of labeled cDNAs to only the segments from which they were derived. These results show that the coding capacity of the dsRNA virus may be at least three times greater than that estimated earlier and illustrates further the complexity of this virus-parasite interrelationship.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Demes P., Gombosová A., Valent M., Yánoska A., Fabusová H., Kasmala L., Garza G. E., Metcalfe E. C. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987 May;55(5):1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Neale K. A. Relatedness of structures of a major immunogen in Trichomonas vaginalis isolates. Infect Immun. 1989 Jun;57(6):1849–1853. doi: 10.1128/iai.57.6.1849-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo R., Engbring J., Alderete J. F. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992 Apr;6(7):853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dailey D. C., Alderete J. F. The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly repeated immunodominant epitope. Infect Immun. 1991 Jun;59(6):2083–2088. doi: 10.1128/iai.59.6.2083-2088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Furfine E. S., White T. C., Wang A. L., Wang C. C. A single-stranded RNA copy of the Giardia lamblia virus double-stranded RNA genome is present in the infected Giardia lamblia. Nucleic Acids Res. 1989 Sep 25;17(18):7453–7467. doi: 10.1093/nar/17.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker M. W., Alderete J. F. Properties of Trichomonas vaginalis grown under chemostat controlled growth conditions. Genitourin Med. 1990 Jun;66(3):193–199. doi: 10.1136/sti.66.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche S., Roth C., Manohar S. K., Dollet M., Baltz T. RNA virus-like particles in pathogenic plant trypanosomatids. Mol Biochem Parasitol. 1993 Feb;57(2):261–267. doi: 10.1016/0166-6851(93)90202-9. [DOI] [PubMed] [Google Scholar]
- Nuss D. L. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol Rev. 1992 Dec;56(4):561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L. Viruses of protozoan parasites. Exp Parasitol. 1990 Jan;70(1):111–113. doi: 10.1016/0014-4894(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revets H., Dekegel D., Deleersnijder W., De Jonckheere J., Peeters J., Leysen E., Hamers R. Identification of virus-like particles in Eimeria stiedae. Mol Biochem Parasitol. 1989 Oct;36(3):209–215. doi: 10.1016/0166-6851(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Schuster F. L. Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool. 1969 Nov;16(4):724–727. doi: 10.1111/j.1550-7408.1969.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251–263. doi: 10.1146/annurev.mi.45.100191.001343. [DOI] [PubMed] [Google Scholar]
- Wang A., Wang C. C., Alderete J. F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med. 1987 Jul 1;166(1):142–150. doi: 10.1084/jem.166.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks R. S., Patterson J. L., Stuart K., Widmer G. Transcribing and replicating particles in a double-stranded RNA virus from Leishmania. Mol Biochem Parasitol. 1992 Jun;52(2):207–213. doi: 10.1016/0166-6851(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Weeks R., Aline R. F., Jr, Myler P. J., Stuart K. LRV1 viral particles in Leishmania guyanensis contain double-stranded or single-stranded RNA. J Virol. 1992 Mar;66(3):1389–1393. doi: 10.1128/jvi.66.3.1389-1393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]