Abstract
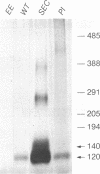
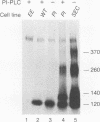
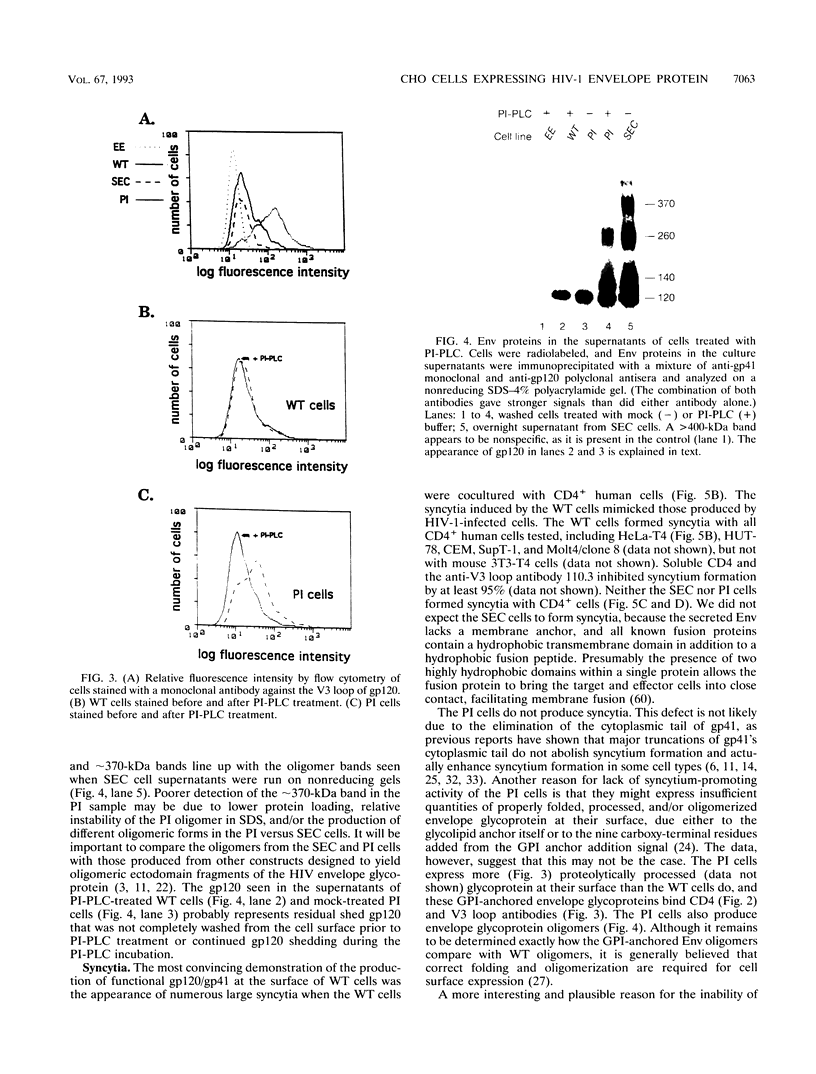
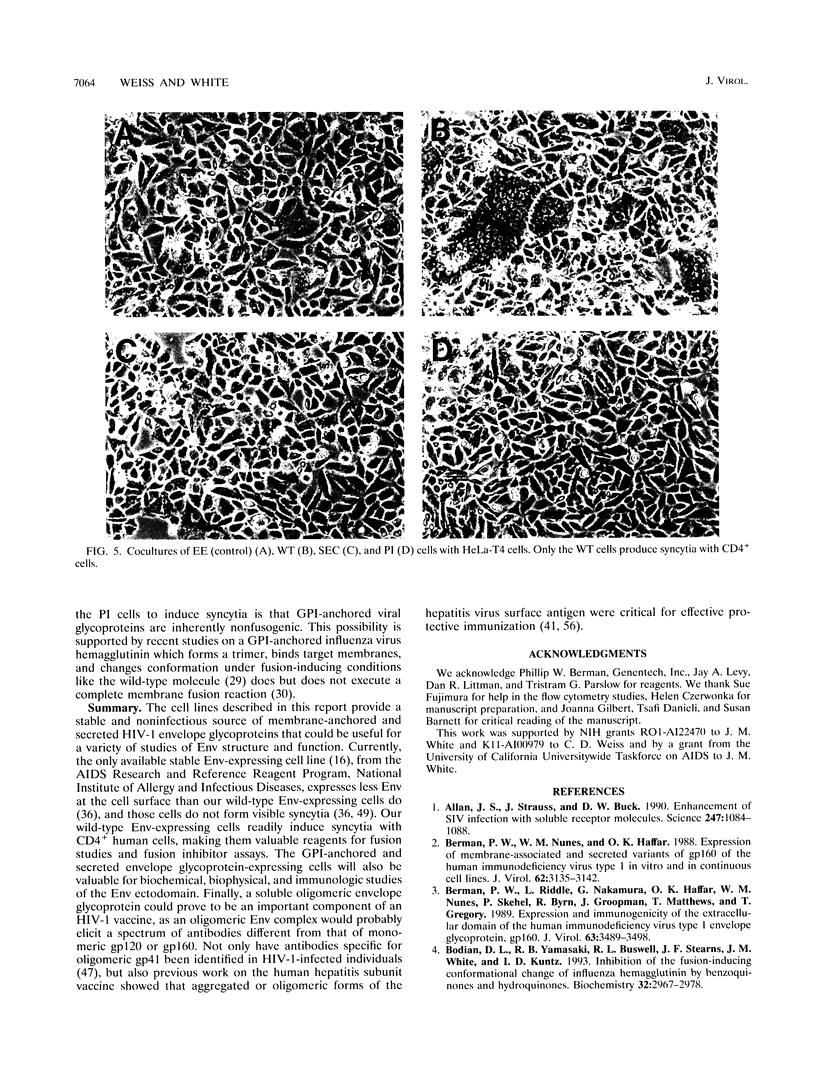
We generated Chinese hamster ovary cell lines that stably express wild-type, secreted, and glycosylphosphatidylinositol (GPI)-anchored envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1). The cells expressing wild-type Env (WT cells) express both the precursor gp160 and the mature gp120/gp41 and readily form large syncytia when cocultivated with CD4+ human cells. The cells expressing secreted Env (SEC cells) release 140-kDa precursor and mature 120-kDa envelope glycoproteins into the supernatants. The cells expressing GPI-anchored Env (PI cells) express both 140-kDa precursor and mature gp120/gp41 envelope glycoproteins, which can be released from the cell surface by treatment with phosphatidylinositol-specific phospholipase C (PI-PLC). Both the secreted and PI-PLC-released envelope glycoproteins form oligomers that can be detected on nonreducing sodium dodecyl sulfate-polyacrylamide gels. In contrast to the WT cells, the SEC and PI cells do not form syncytia when cocultivated with CD4+ human cells. The availability of cells producing water-soluble oligomers of HIV-1 Env should facilitate studies of envelope glycoprotein structure and function. The WT cells, which readily induce syncytia with CD4+ cells, provide a convenient system for assessing potential fusion inhibitors and for studying the fusion mechanism of the HIV Env glycoprotein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Nunes W. M., Haffar O. K. Expression of membrane-associated and secreted variants of gp160 of human immunodeficiency virus type 1 in vitro and in continuous cell lines. J Virol. 1988 Sep;62(9):3135–3142. doi: 10.1128/jvi.62.9.3135-3142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Riddle L., Nakamura G., Haffar O. K., Nunes W. M., Skehel P., Byrn R., Groopman J., Matthews T., Gregory T. Expression and immunogenicity of the extracellular domain of the human immunodeficiency virus type 1 envelope glycoprotein, gp160. J Virol. 1989 Aug;63(8):3489–3498. doi: 10.1128/jvi.63.8.3489-3498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodian D. L., Yamasaki R. B., Buswell R. L., Stearns J. F., White J. M., Kuntz I. D. Inhibition of the fusion-inducing conformational change of influenza hemagglutinin by benzoquinones and hydroquinones. Biochemistry. 1993 Mar 30;32(12):2967–2978. doi: 10.1021/bi00063a007. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Mordenti J., Lucas C., Smith D., Marsters S. A., Johnson J. S., Cossum P., Chamow S. M., Wurm F. M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990 Apr 12;344(6267):667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Emerman M., Tiollais P., Sonigo P. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J Virol. 1989 Oct;63(10):4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crise B., Ruusala A., Zagouras P., Shaw A., Rose J. K. Oligomerization of glycolipid-anchored and soluble forms of the vesicular stomatitis virus glycoprotein. J Virol. 1989 Dec;63(12):5328–5333. doi: 10.1128/jvi.63.12.5328-5333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Duval N., Krejci E., Grassi J., Coussen F., Massoulié J., Bon S. Molecular architecture of acetylcholinesterase collagen-tailed forms; construction of a glycolipid-tailed tetramer. EMBO J. 1992 Sep;11(9):3255–3261. doi: 10.1002/j.1460-2075.1992.tb05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Doms R. W., Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990 Jan;87(2):648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D. H., Lever A., Terwilliger E., Sodroski J. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992 Jun;66(6):3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Reupke H., Pauli G. Loss of envelope antigens of HTLV-III/LAV, a factor in AIDS pathogenesis? Lancet. 1985 Nov 2;2(8462):1016–1017. doi: 10.1016/s0140-6736(85)90570-7. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Hernandez L. D., Chernov-Rogan T., White J. M. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J Virol. 1993 Nov;67(11):6889–6892. doi: 10.1128/jvi.67.11.6889-6892.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F., Waxham M. N., Ross A. M., Hoxie J. A. Sequence similarities between human immunodeficiency virus gp41 and paramyxovirus fusion proteins. AIDS Res Hum Retroviruses. 1987 Fall;3(3):245–252. doi: 10.1089/aid.1987.3.245. [DOI] [PubMed] [Google Scholar]
- Haffar O. K., Dowbenko D. J., Berman P. W. Topogenic analysis of the human immunodeficiency virus type 1 envelope glycoprotein, gp160, in microsomal membranes. J Cell Biol. 1988 Nov;107(5):1677–1687. doi: 10.1083/jcb.107.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O. K., Nakamura G. R., Berman P. W. The carboxy terminus of human immunodeficiency virus type 1 gp160 limits its proteolytic processing and transport in transfected cell lines. J Virol. 1990 Jun;64(6):3100–3103. doi: 10.1128/jvi.64.6.3100-3103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger S., Tucker S. P., Owens R. J., Bernstein H. B., Compans R. W. Secretion of a truncated form of the human immunodeficiency virus type 1 envelope glycoprotein. Virology. 1993 Mar;193(1):510–514. doi: 10.1006/viro.1993.1156. [DOI] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Gabuzda D., Ardman B., Haseltine W., Sodroski J. Changes in the transmembrane region of the human immunodeficiency virus type 1 gp41 envelope glycoprotein affect membrane fusion. J Virol. 1990 Dec;64(12):6314–6318. doi: 10.1128/jvi.64.12.6314-6318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Hope T. J., McDonald D., Huang X. J., Low J., Parslow T. G. Mutational analysis of the human immunodeficiency virus type 1 Rev transactivator: essential residues near the amino terminus. J Virol. 1990 Nov;64(11):5360–5366. doi: 10.1128/jvi.64.11.5360-5366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kemble G. W., Bodian D. L., Rosé J., Wilson I. A., White J. M. Intermonomer disulfide bonds impair the fusion activity of influenza virus hemagglutinin. J Virol. 1992 Aug;66(8):4940–4950. doi: 10.1128/jvi.66.8.4940-4950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble G. W., Henis Y. I., White J. M. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol. 1993 Sep;122(6):1253–1265. doi: 10.1083/jcb.122.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiro S., Imai M., Takahashi K., Machida A., Gotanda T., Miyakawa Y., Mayumi M. A 49,000-dalton polypeptide bearing all antigenic determinants and full immunogenicity of 22-nm hepatitis B surface antigen particles. J Immunol. 1980 Apr;124(4):1589–1593. [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moran P., Caras I. W. Fusion of sequence elements from non-anchored proteins to generate a fully functional signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991 Dec;115(6):1595–1600. doi: 10.1083/jcb.115.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. A., Stearns S. M., Littman D. R. Analysis of mutations in the V3 domain of gp160 that affect fusion and infectivity. J Virol. 1992 Jan;66(1):524–533. doi: 10.1128/jvi.66.1.524-533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Price T. M. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988 Sep;107(3):959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L. G., O'Donnell M. A., Stephens E. B. The transmembrane glycoprotein of human immunodeficiency virus type 1 induces syncytium formation in the absence of the receptor binding glycoprotein. J Virol. 1992 Jul;66(7):4134–4143. doi: 10.1128/jvi.66.7.4134-4143.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A., Bona C., Zaghouani H., Gorny M. K., Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht R. M., Bernard L. D., Bronson R., Gama Sosa M. A., Mullaney S. Castanospermine vs. its 6-O-butanoyl analog: a comparison of toxicity and antiviral activity in vitro and in vivo. J Acquir Immune Defic Syndr. 1991;4(1):48–55. [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawaller M., Smith G. E., Skehel J. J., Wiley D. C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989 Sep;172(1):367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- Sinangil F., Loyter A., Volsky D. J. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988 Oct 24;239(1):88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- Singh I., Doms R. W., Wagner K. R., Helenius A. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 1990 Mar;9(3):631–639. doi: 10.1002/j.1460-2075.1990.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M. A., DeGasperi R., Fazely F., Ruprecht R. M. Human cell lines stably expressing HIV env and tat gene products. Biochem Biophys Res Commun. 1989 May 30;161(1):305–311. doi: 10.1016/0006-291x(89)91597-0. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Thanh L. T., Man N. T., Mat B., Tran P. N., Ha N. T., Morris G. E. Structural relationships between hepatitis B surface antigen in human plasma and dimers from recombinant vaccine: a monoclonal antibody study. Virus Res. 1991 Oct;21(2):141–154. doi: 10.1016/0168-1702(91)90004-f. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Wall J. S., Hainfeld J. F., Kaczorek M., Booy F. P., Trus B. L., Eiserling F. A., Steven A. C. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991 Jul;65(7):3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C. D., Levy J. A., White J. M. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J Virol. 1990 Nov;64(11):5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein D. A., Boniface J. J., Reay P. A., Schild H., Davis M. M. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J Exp Med. 1991 Jul 1;174(1):219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J. M., Wilson I. A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987 Dec;105(6 Pt 2):2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]