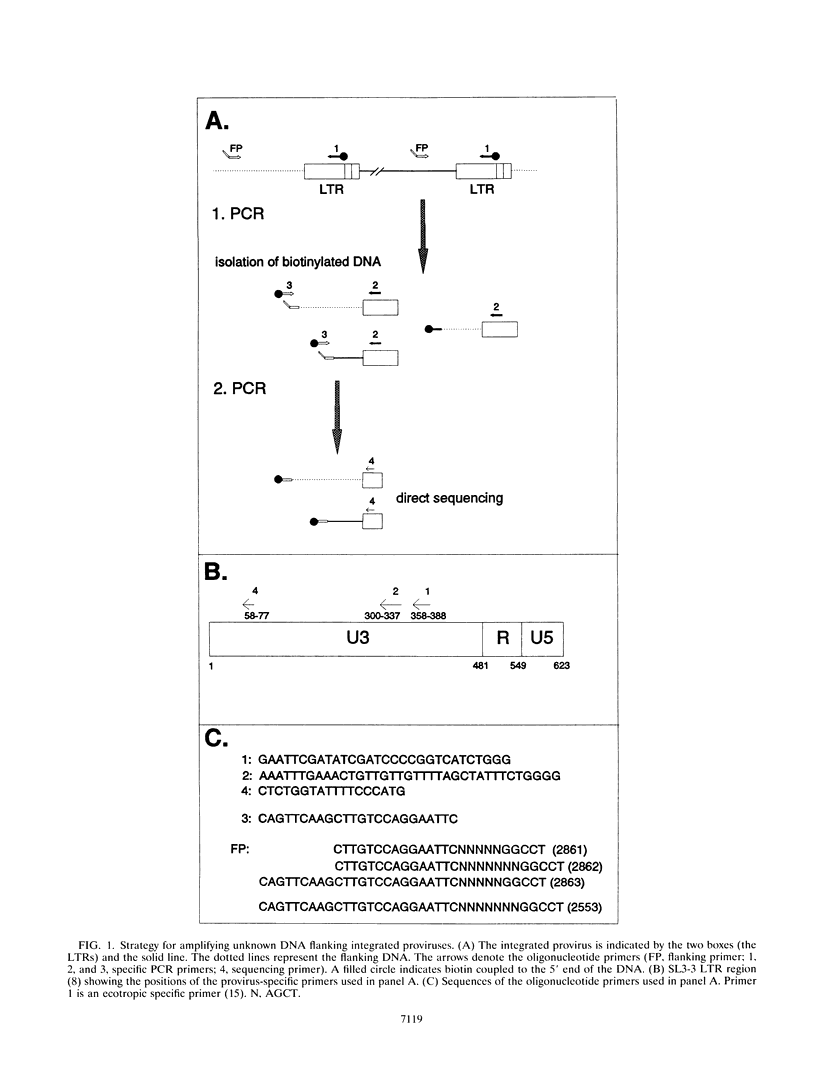
Abstract
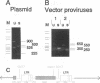
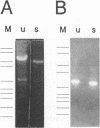
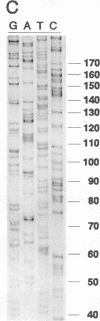
We describe a two-step polymerase chain reaction method that can be used for the amplification of cellular DNA sequences adjacent to an integrated retroviral provirus. The technique involves a partly degenerate, arbitrary primer that will hybridize in the provirus-flanking cellular DNA. By using this primer in combination with a biotinylated provirus-specific primer, a provirus-cellular DNA junction fragment can be isolated from the nonspecific amplification products by using streptavidin-coated magnetic beads. A second amplification employing a nested provirus-specific primer and a biotinylated nondegenerate primer derived from the partly degenerate primer followed by purification with streptavidin-coated beads enhances the specificity and the efficiency of recovery of a fragment(s) containing the unknown flanking sequences. In addition to being relevant in studies of viral integration sites, the method should be generally useful to analyze DNA sequences either upstream or downstream from a known sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Etzerodt M., Mikkelsen T., Pedersen F. S., Kjeldgaard N. O., Jørgensen P. The nucleotide sequence of the Akv murine leukemia virus genome. Virology. 1984 Apr 15;134(1):196–207. doi: 10.1016/0042-6822(84)90285-x. [DOI] [PubMed] [Google Scholar]
- Hallberg B., Schmidt J., Luz A., Pedersen F. S., Grundström T. SL3-3 enhancer factor 1 transcriptional activators are required for tumor formation by SL3-3 murine leukemia virus. J Virol. 1991 Aug;65(8):4177–4181. doi: 10.1128/jvi.65.8.4177-4181.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
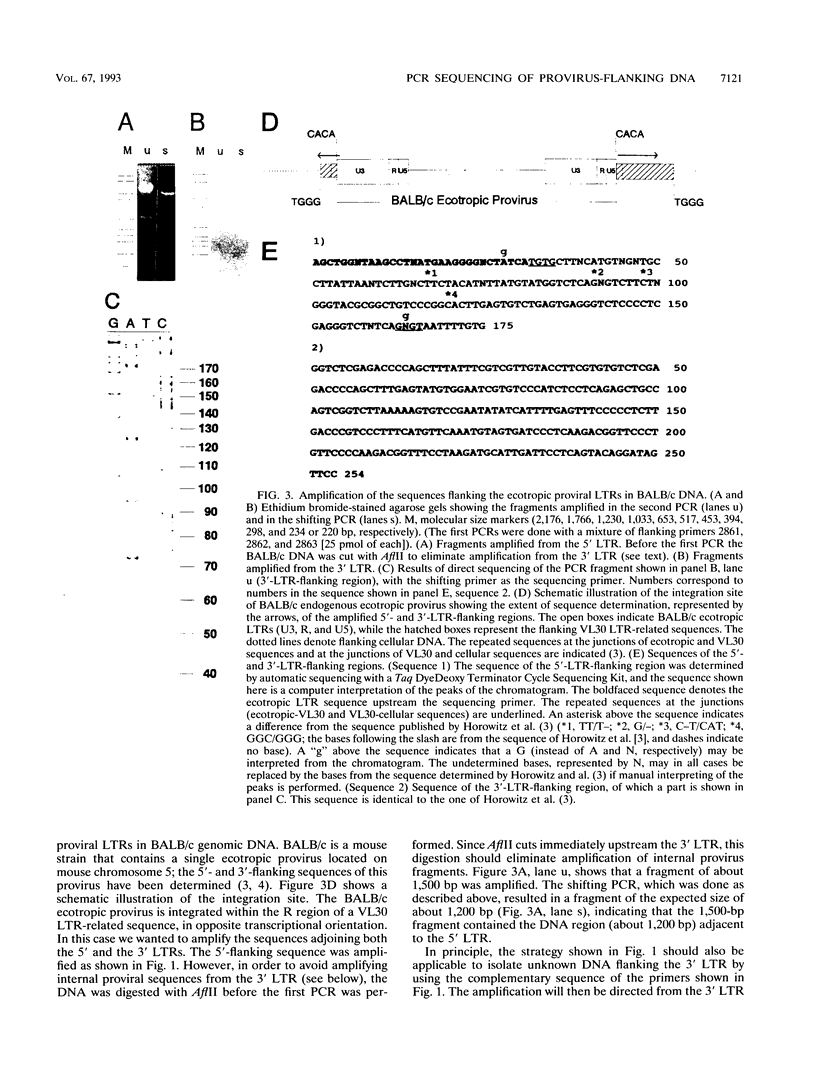
- Horowitz J. M., Holland G. D., King S. R., Risser R. Germ line integration of a murine leukemia provirus into a retroviruslike sequence. J Virol. 1987 Mar;61(3):701–707. doi: 10.1128/jvi.61.3.701-707.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. Molecular and biological characterization of the endogenous ecotropic provirus of BALB/c mice. J Virol. 1985 Dec;56(3):798–806. doi: 10.1128/jvi.56.3.798-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N. A., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Mammalian expression-and-transmission vector derived from Akv murine leukemia virus. Gene. 1986;41(1):59–65. doi: 10.1016/0378-1119(86)90267-2. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Winistorfer S. C. Sequence specific generation of a DNA panhandle permits PCR amplification of unknown flanking DNA. Nucleic Acids Res. 1992 Feb 11;20(3):595–600. doi: 10.1093/nar/20.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan K., Dai H. Y., Duch M., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Different relative expression from two murine leukemia virus long terminal repeats in unintegrated transfected DNA and in integrated retroviral vector proviruses. J Virol. 1989 Dec;63(12):5201–5207. doi: 10.1128/jvi.63.12.5201-5207.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. D., Rabinovitch P. S., Burmer G. C. Targeted gene walking polymerase chain reaction. Nucleic Acids Res. 1991 Jun 11;19(11):3055–3060. doi: 10.1093/nar/19.11.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J., Butler R., Ogilvie D., Finniear R., Jenner D., Powell S., Anand R., Smith J. C., Markham A. F. A novel, rapid method for the isolation of terminal sequences from yeast artificial chromosome (YAC) clones. Nucleic Acids Res. 1990 May 25;18(10):2887–2890. doi: 10.1093/nar/18.10.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Jones D. S. Genomic walking and sequencing by oligo-cassette mediated polymerase chain reaction. Nucleic Acids Res. 1990 May 25;18(10):3095–3096. doi: 10.1093/nar/18.10.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Silver J., Keerikatte V. Novel use of polymerase chain reaction to amplify cellular DNA adjacent to an integrated provirus. J Virol. 1989 May;63(5):1924–1928. doi: 10.1128/jvi.63.5.1924-1928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell A., Hallberg B., Grundström T. Binding of SL3-3 enhancer factor 1 transcriptional activators to viral and chromosomal enhancer sequences. J Virol. 1991 Jan;65(1):42–50. doi: 10.1128/jvi.65.1.42-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]