Abstract
Chronic Helicobacter pylori infection is recognized as a cause of gastric cancer. H. pylori adhesion to gastric cells is mediated by bacterial adhesins such as sialic acid–binding adhesin (SabA), which binds the carbohydrate structure sialyl–Lewis x. Sialyl–Lewis x expression in the gastric epithelium is induced during persistent H. pylori infection, suggesting that H. pylori modulates host cell glycosylation patterns for enhanced adhesion. Here, we evaluate changes in the glycosylation-related gene expression profile of a human gastric carcinoma cell line following H. pylori infection. We observed that H. pylori significantly altered expression of 168 of the 1,031 human genes tested by microarray, and the extent of these alterations was associated with the pathogenicity of the H. pylori strain. A highly pathogenic strain altered expression of several genes involved in glycan biosynthesis, in particular that encoding β3 GlcNAc T5 (β3GnT5), a GlcNAc transferase essential for the biosynthesis of Lewis antigens. β3GnT5 induction was specific to infection with highly pathogenic strains of H. pylori carrying a cluster of genes known as the cag pathogenicity island, and was dependent on CagA and CagE. Further, β3GnT5 overexpression in human gastric carcinoma cell lines led to increased sialyl–Lewis x expression and H. pylori adhesion. This study identifies what we believe to be a novel mechanism by which H. pylori modulates the biosynthesis of the SabA ligand in gastric cells, thereby strengthening the epithelial attachment necessary to achieve successful colonization.
Introduction
Gastric carcinoma is a highly incident disease and the second leading cause of cancer-related deaths worldwide (1). The gastric carcinogenesis pathway follows a sequence of events in which chronic infection with Helicobacter pylori (Hp) is a risk factor for gastric cancer. Hp is a Gram-negative, spiral-shaped microaerophilic bacteria that is involved in several gastric diseases. Individuals infected by Hp develop gastritis, and up to 10% of infected individuals develop duodenal ulcer disease. Persistent infection with Hp may cause chronic atrophic gastritis, with development of intestinal metaplasia, dysplasia, and gastric carcinoma (2–5). This carcinogenesis pathway is reinforced by experiments in animal models that show that Hp induces gastritis, intestinal metaplasia, and gastric carcinoma (6–8). In 1994, the International Agency for Research on Cancer classified Hp as a carcinogenic agent class I.
The mechanism of Hp pathogenicity is not well understood, although bacterial virulence and host susceptibility factors have been associated with the development of chronic gastric inflammation and gastric carcinogenesis (4, 9, 10). Hp is a genetically heterogeneous species, with strains differing markedly in virulence. Several bacterial genetic loci have been associated with the development of malignancy, among them the cag pathogenicity island (cagPAI), a cluster of genes that encodes a type IV secretion system through which bacterial proteins are injected into the host cells; the vacA gene, which encodes a vacuolating cytotoxin; and the babA2 gene, codifying a bacterial adhesin. These bacterial genes are associated with the risk of development of gastric disease and are used as markers of virulence (11, 12).
The first step for a successful infection of the gastric mucosa is the adhesion of Hp to the epithelial cells. The adhesion of Hp is mediated by bacterial adhesins that bind carbohydrate ligands expressed by the epithelial gastric cells. The babA2 gene encodes the adhesin BabA, which is a member of a family of highly conserved, strain-specific outer membrane proteins that bind to the Lewis b (Leb) histo-blood group antigen and H type 1 carbohydrate structures expressed on gastric mucins (13). Hp strains that possess the babA2 gene are associated with an increased risk for gastric adenocarcinoma (11). In addition, Hp has been shown to adhere to sialylated glycoconjugates expressed during chronic inflammation, and a sialic acid–binding adhesin (SabA) has been identified and demonstrated to bind the carbohydrate structure sialyl-Lex (14). The sialyl-Lex antigen expression in the gastric epithelium is induced during persistent Hp infection, suggesting that Hp may trigger the host tissue to re-tailor the gastric mucosal glycosylation patterns to express ligands for bacterial adhesins (14).
The biosynthesis of carbohydrate chains on glycoproteins and glycolipids expressed by gastric cells is controlled and regulated by glycosyltransferases. These enzymes transfer the sugar moiety from a nucleotide sugar to an acceptor, which may be a growing oligosaccharide on a lipid or a protein. There are several families of glycosyltransferase genes identified, including N-acetylgalactosaminyltransferases, galactosyltransferases, fucosyltransferases, and sialyltransferases, among others. Each of these glycosyltransferases show donor and acceptor substrate specificities (15) and constitute the primary basis for determining the structures of the oligosaccharides produced by a cell. The complexity of the biosynthesis of carbohydrate structures that may function as ligands for bacterial adhesins requires a large-scale analysis of gene expression. Although complex glycans are, so far, the known ligands for Hp adhesins, the expression of human genes involved in carbohydrate chain biosynthesis in response to Hp infection remains largely unknown.
In this context, we first analyzed the gene expression profile of human gastric carcinoma cell line MKN45 infected with 2 different strains of Hp: the high-pathogenicity 26695 strain (high-Hp) and the low-pathogenicity Tx30a strain (low-Hp). The expression levels were assessed using the Consortium of Functional Glycomics GLYCOv2 glycogene array, which contains probe sets to monitor the expression of 1,031 human transcripts and has been developed using Affymetrix technology. This array includes different classes of genes involved in the biosynthesis of carbohydrates, as well as glycan-binding proteins, transporter proteins, mucins, growth factors, cytokines, and chemokines, among others. We evaluated the significant expression alterations from the 1,031 genes tested and analyzed whether the altered genes were related with the pathogenicity of Hp strains. We confirmed the alterations observed in genes related with glycosylation and identified a specific glycosyltransferase, β3 GlcNAc T5 (β3GnT5), described to be involved in the biosynthesis pathway of carbohydrate chains on glycolipids, such as sialyl-Lex (16–18). Using 2 different gastric cell lines, we searched for associations between this glycosyltransferase and the cagPAI status of 14 different Hp strains. Finally, we overexpressed β3GnT5 and demonstrated its role in the biosynthesis of terminal Lewis antigens, namely sialyl-Lex, and its effect on Hp adhesion to gastric cells. Our results provide valuable information about the molecular mechanisms underlying the pathogenesis of Hp.
Results
Modulation of gene expression profiles in gastric cell line induced by Hp strains.
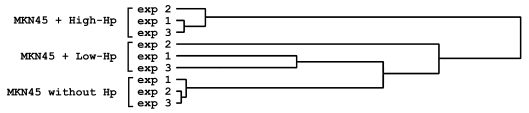
Differences in gene expression in human gastric MKN45 cells infected with either 26695 or Tx30a strains (high-Hp and low-Hp, respectively) were analyzed. In order to evaluate the relationship between sample data sets in the experiment, an ANOVA test was performed using all samples from the 3 groups (cells without Hp, infected with high-Hp and infected with low-Hp). This analysis yielded 127 genes found to be significantly differentially expressed in at least 1 of the 3 groups. This list of 127 genes was used to create a supervised cluster and heat map, as shown in Figure 1 and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI34324DS1). By analyzing the centered correlation and average linkage dendrogram, we observed a distinct separation of the groups in the clustering for all the treatment groups (Figure 1). Differences among treatment groups were much larger than differences observed between replicates (within groups), allowing for statistical confidence in identifying genes that differentiate between classes. Only the low-Hp treatment group showed higher heterogeneity between samples, with 1 replicate clustering away from its expected group. All 3 low-Hp samples clustered closer to the control group than to the high-Hp treatment group.
Figure 1. Supervised hierarchical clustering analysis of genes expressed by MKN45 gastric cell line cultured with high-Hp, low-Hp, and without Hp.
Cluster was created using only 127 genes determined as significantly differentially expressed by ANOVA (see Methods). The dendrogram was created using centered correlation and average linkage.
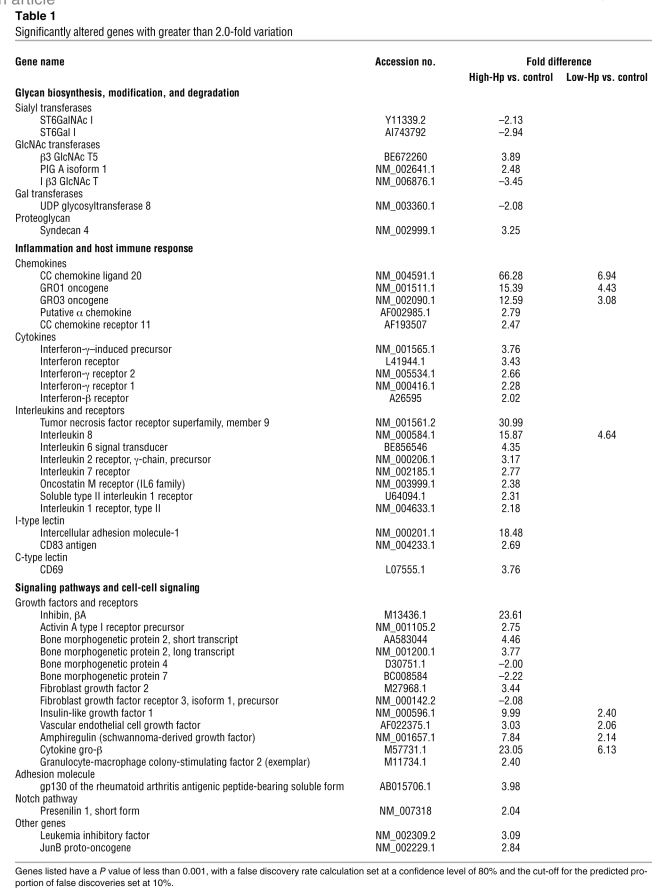
Class comparison data analysis revealed that 168 genes were significantly altered by infection with the high-Hp (as described in Methods; Supplemental Tables 1–3). Differences in gene expression ranged from a 66.3-fold upregulation to a 3.5-fold downregulation. From these 168 genes, 38 had fold increases above 2.0, and 7 genes had fold decreases below 2.0-fold.
Infection with low-Hp strain yielded 49 altered genes, which ranged from a 6.9-fold upregulation to a 1.5-fold downregulation. From these 49 genes, 8 were increased more than 2.0-fold.
Noteworthy, all genes that were markedly altered by incubation with the low-Hp strain were also altered by high-Hp strain, though with a higher fold difference (Supplemental Tables 1–3).
Only genes whose expression was significantly altered beyond the arbitrary 2.0-fold cut-off were considered for further discussion and are presented in Table 1.
Table 1 .
Significantly altered genes with greater than 2.0-fold variation
Differentially expressed genes by Hp infection of gastric cells.
Hp induced the most substantial alterations in genes involved in the inflammatory process and in the activation of immune response. All genes within this group showed only increases in expression (Table 1).
Several alterations in expression of growth factors and receptors were also induced by Hp, revealing that various signaling pathways may be modified. One such pathway is the TGF-β pathway, which showed various genes with altered expression levels, namely increases in expression of inhibin βA, activin A type I receptor precursor, and BMP2. On the other hand, high-Hp induced a decreased expression of BMP4 and BMP7. Low-Hp also exerted some mild alterations in terms of fold difference when compared with high-Hp. Interestingly, all genes altered by low-Hp were also altered by high-Hp to a higher extent and were not a different subset of genes. This observation credits responsibility for the alterations on the pathogenicity of each strain.
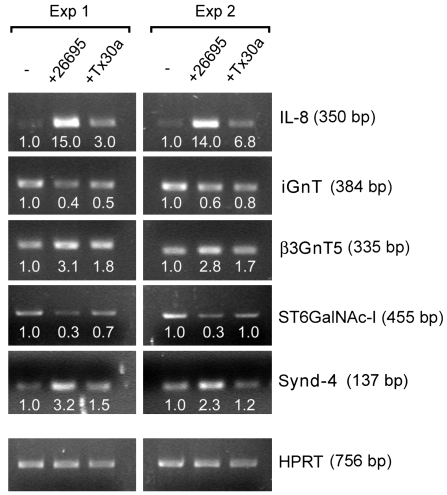
Hp induced alterations in the expression levels of several genes involved in the biosynthesis of glycoconjugates. Expression of sialyltransferases ST6GalNac-I and ST6Gal-I was reduced by the presence of high-Hp (Table 1 and Figure 2). High-Hp also induced a greater than 2-fold variation in expression of glycosyltransferases involved in the biosynthesis of the blood group neolactoseries and lactoseries of glycolipids. As shown in Table 1, β3GnT5 showed a 3.9-fold increased expression, whereas I β3 GlcNAc T (iGnT) showed a 3.5-fold reduced expression. The high-Hp also induced alterations in other glycosyltransferase genes, including the PIG A isoform 1 and UGT8. Low-Hp exerted no alterations above 2.0-fold in any of the genes related to glycan biosynthesis and modification.
Figure 2. RT-PCR confirmation of mRNA expression of selected genes in uninfected MKN45 cells (–) or infected with either high-Hp or low-Hp.
Two independent experiments are shown (Exp. 1 and Exp. 2). Densitometry was performed on gel bands using Quantity One software (BioRad) and normalized to expression of the HRPT gene. Values are presented below each gel band as fold increase relative to the respective control (–).
Relationship between Hp cagPAI status and β3GnT5 expression.
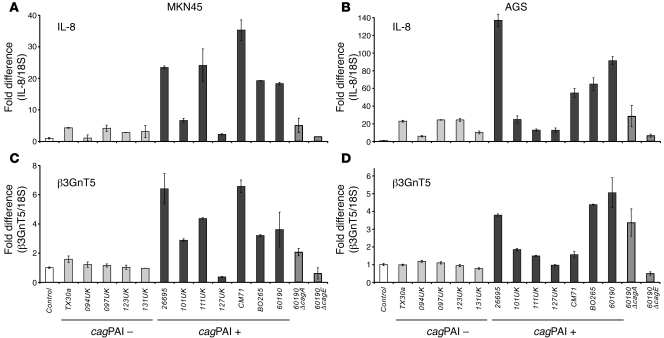
We further addressed the effect of strain pathogenicity on β3GnT5 expression using a panel of 5 cagPAI– and 7 cagPAI+ Hp strains to infect 2 gastric cell lines, MKN45 and AGS. β3GnT5 is a GlcNAc transferase that has been described to be essential for the initiation of lactoseries (type 1) and neolactoseries (type 2) on glycolipids (18). These structures are precursors for the formation of the different Lewis antigens, namely those that function as ligands for Hp adhesins. Using quantitative real-time PCR, IL-8 expression was measured as a reporter gene of infection. The majority of cagPAI+ Hp strains led to an increase of this proinflammatory molecule, whereas cagPAI– strains led to minor or no increases (Figure 3, A and B), as expected. Strain 127UK was an exception, since, although it is described as a cagPAI+ strain, it induced very low levels of IL-8 expression, similar to cagPAI– strains. Similarly, we observed that only the cagPAI+ Hp strains induce β3GnT5 expression (Figure 3, C and D), again with the exception of 127UK strain. There were no significant differences between β3GnT5 expression levels in cells infected with cagPAI– strains when compared with uninfected gastric cells (control), showing that β3GnT5 is upregulated specifically by pathogenic Hp strains.
Figure 3. Analysis of IL-8 (A and B) and β3GnT5 (C and D) gene expression in MKN45 (A and C) and AGS (B and D) cells without Hp (control) or infected with different Hp strains.
Real-time PCR reactions were performed in triplicate. Target gene expressions levels were normalized to 18S expression levels, and results are presented as fold differences relative to uninfected cells.
Analysis of β3GnT5 expression in cells infected with strain 60190 isogenic mutants for cagA and cagE showed significant alterations when compared with cells infected with the parental strain (Figure 3, C and D). Infection with strain 60190ΔcagA led to a decrease in β3GnT5 expression in both cell lines, indicating a role for CagA in the modulation of β3GnT5 induction. The cagE mutant completely ablated the induction of this enzyme, inducing lower levels of β3GnT5 than the WT 60190 strain or the cagA mutant.
Proinflammatory cytokines and β3GnT5 expression.
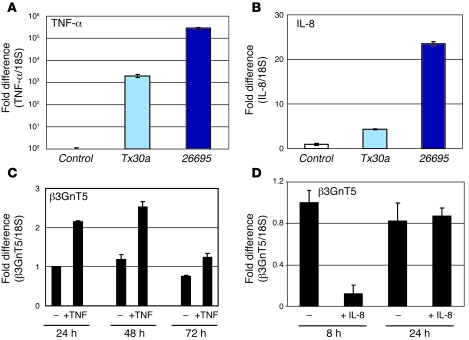
TNF-α has been shown to stimulate increased expression of several sialyl-, fucosyl-, and sulfotransferases (19). We evaluated the mRNA expression of TNF-α in MKN45 cells infected with Tx30a and 26695 strains and observed that TNF-α was barely detectable in uninfected cells. Both strains induced a remarkable increase in TNF-α expression, with the cagPAI+ strain inducing about 100-fold greater expression than the cagPAI– strain (Figure 4A). Addition of TNF-α to culture medium at a concentration of 40 ng/ml led to a 2.2-fold increase of β3GnT5 after 24 h, a 2.1-fold increase after 48 h, and a 1.7-fold increase after 72 h when compared with their respective negative controls (Figure 4C).
Figure 4. Cytokine expression and β3GnT5 induction.
Analysis by real-time PCR of mRNA expression of TNF-α (A) and IL-8 (B) in MKN45 cells without Hp (control) or infected with low-Hp or high-Hp strains. Real-time PCR of β3GnT5 mRNA expression after 24 h, 48 h, and 72 h in the absence (–) or presence of 40 ng/ml TNF-α (C) or after 8 h and 24 h in the absence or presence of 50 ng/ml IL-8 (D). Real-time PCR reactions were performed in triplicate. Target gene expression levels were normalized to 18S expression levels, and results are presented as fold differences compared with untreated cells.
Similar to the microarray analysis, quantitative real-time PCR evaluation of IL-8 in MKN45 cells showed that 26695 strain led to a 23.5-fold increase, whereas Tx30a led to a 4.3-fold increase (Figure 4B). Addition of IL-8 to culture medium at a concentration of 50 ng/ml led to no variation of β3GnT5 after 24 h, but at 8 h, the time point used for the infection studies, an 8.3-fold decrease was observed (Figure 4D).
Stable transfection of human β3GnT5 in gastric cell lines.
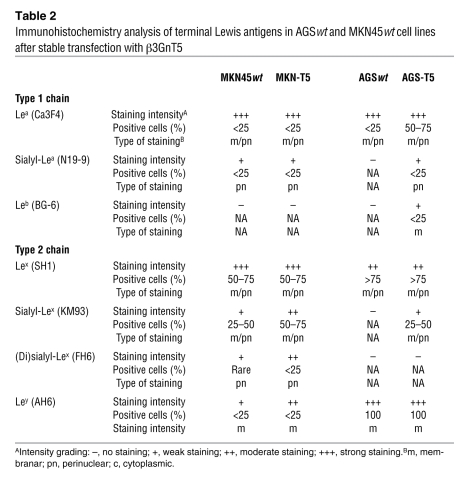
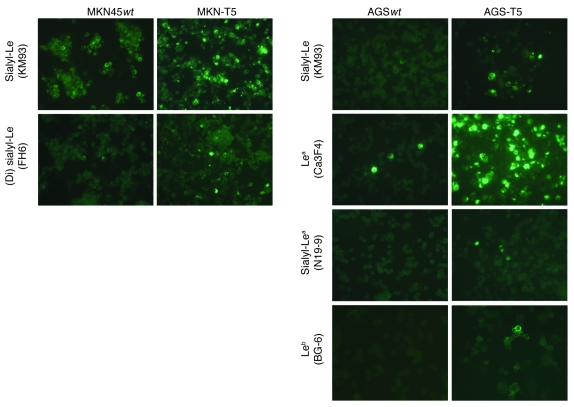
To better understand the role of β3GnT5 in the biosynthesis of Lewis antigens, MKN45 and AGS cells were stably transfected with the full-length human β3GnT5 (renamed MKN-T5 and AGS-T5, respectively). Transfection led to increased β3GnT5 mRNA expression of 2.9-fold and 1.9-fold in MKN-T5 and AGS-T5, respectively, when compared with mock-transfected cells (Supplemental Figure 2). Immunocytochemistry assays showed that β3GnT5 overexpression led to alterations in the glycosylation profile of both cell lines (Table 2 and Figure 5). We observed an upregulation of sialyl-Lex antigen expression in both MKN-T5 and AGS-T5 cells, as detected by KM93 mAb, when compared with mock-transfected and WT cells. MKN-T5 also showed an overexpression of (di)sialyl-Lex, detected by mAb FH6. Other Lewis antigens were also analyzed, and Lea, sialyl-Lea, Leb, Lex, and Ley antigens were not altered in MKN-T5. AGS-T5 showed a strong increase in expression of Lea and a weak increase in sialyl-Lea and Leb (Table 2 and Figure 5). Mock-transfected cells showed the same results as WT cells in all assays (data not shown).
Table 2 .
Immunohistochemistry analysis of terminal Lewis antigens in AGSwt and MKN45wt cell lines after stable transfection with β3GnT5
Figure 5. Immunofluorescence detecting terminal Lewis antigens.
Representative micrographs of Lewis antigens in AGS-T5 and MKN-T5 cells that showed alterations when compared with their parental counterparts. Original magnification, ×400.
Characterization of Hp adhesion by in vitro binding to gastric cell lines.
In order to assess the relevance of the overexpression of sialyl-Lex in the process of Hp adherence, in vitro bacterial adherence assays were performed with MKN45 and MKN-T5 cells.
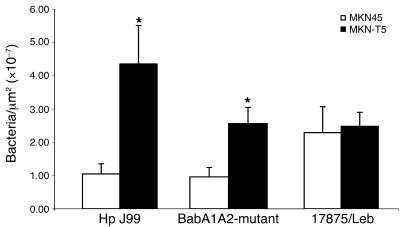
Hp strain 17875/Leb, which only binds Leb-related antigens and does not bind to sialyl-Lex, showed a similar profile in binding affinities for the MKN45 and MKN-T5 cells lines. On the other hand, the 17875 isogenic babA1A2 mutant, which lacks Leb binding activity but binds to sialyl-Lex, and J99 (which binds both structures) showed more binding to MKN-T5 cells expressing sialyl-Lex in comparison with WT counterparts (Figure 6). Mock-transfected cells showed the same results as WT cells in all adhesion assays (data not shown).
Figure 6. Analysis of adhesion of the Hp strains 17875/Leb, babA1A2-mutant and J99 to MKN45 and MKN-T5 cells.
Adherence of the Hp strains was scored according to the amount of bound bacteria in the MKN45 and MKN-T5 cell lines. *P < 0.001.
Discussion
The pathogenesis of gastric diseases caused by Hp is not clearly understood, though the virulence factors of the strain, the capability of establishing a chronic infection, persistent inflammation, and host immunologic mechanisms have been shown to be important in the process of gastric carcinogenesis. In the present study, we demonstrate that Hp induces several alterations in gene expression in gastric cells, including induction of a glycosyltransferase that alters glycan structures that promote bacterial adhesion and persistent infection (14, 20).
Microarray analysis of gene expression of a human gastric epithelial cell line showed that gene response to Hp infection was rather narrow and specific for the Hp strain. From the analysis of the expression status of 1,031 human genes, our results showed that high-Hp significantly altered a total of 168 genes, a considerable larger set than low-Hp. Interestingly, all 39 genes altered by low-Hp were also consistently altered by high-Hp strain, but to a higher fold. Figure 1 shows that, when analyzing the significantly differently expressed genes, the low-Hp treatment group clustered closer to the control group (without Hp) and clearly separated from the high-Hp group. These results show that the outcome of an infection with low-Hp is more similar to the basal state of cells alone than to that of cells infected with a highly pathogenic strain. These observations reflect the characteristics of virulence of the high-Hp strain (cagPAI+), showing that the degree of pathogenicity of the different strains is highly related to gene expression alterations induced in host cells. The cagPAI encodes a bacterial type IV secretion system that is involved in the process of activation of AP-1 and NF-κB transcription factors, leading to IL-8 production (21–24). The present study shows that, despite both High- and low-Hp strains increasing the expression levels of small inducible cytokine A20 (CCL20), GRO1, and IL-8, infection with the high-Hp strain containing the cagPAI resulted in greater induction than that observed with the low-Hp strain, therefore confirming the increased pathogenicity attributed to cagPAI+ strains.
A crucial step for chronic Hp infection of the gastric mucosa is the adhesion of Hp to epithelial cells of the gastric mucosa. Bacteria have multiple lectin-type molecules, called adhesins, that display different carbohydrate specificity. The adhesion of Hp is mediated by BabA and SabA that bind to epithelial gastric cells through the Leb- and sialyl-Lex–related carbohydrate antigens, respectively (13, 14). We observed that the highly pathogenic cagPAI+ high-Hp strain adhered to gastric cells in greater numbers, compared with the noncytotoxic low-Hp (data not shown). This observation suggests that the factors of virulence that differ between strains may be responsible for modulating host ligand expression. Control of expression of glycan structures is determined by the concerted action of numerous genes that code for glycosyltransferases, glycosidases, and accessory enzymes involved in the synthesis and transport of nucleotide sugars. The present study shows that both high- and low-pathogenic strains induced alterations in the expression levels of several glycosyltransferase genes, but only high-Hp led to alterations above 2.0-fold (Table 1). This is the case of ST6GalNAc-I, an enzyme responsible for the biosynthesis of the sialyl Tn antigen (25), and ST6Gal-I, responsible for α2,6sialylation of type 2 chains (26). Several GalNAc transferases and nucleotide sugar synthases were significantly altered by high-Hp, although by less than 2.0-fold (Supplemental Tables 1 and 3).
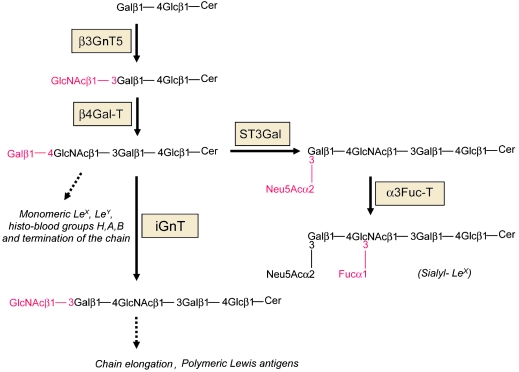
High-Hp induced a 3.9-fold increased expression of β3GnT5. The enhanced expression of β3GnT5 has been shown to lead to accumulation of both type 1 and type 2 carbohydrate chains on glycolipids (18), suggesting that it plays a key role in regulating the level of expression of different antigens on type 1 and type 2 chains. Further substitution by a panel of sialyltransferases and fucosyltransferases will complete the biosynthesis of different antigens (Figure 7). Interestingly, enzymatic studies have shown that this GlcNAc transferase activity is the key step that is missing in normal colonic cells, which are devoid of sialyl-Lex, but this activity is markedly upregulated in colon cancer cells, which express sialyl-Lex (16, 17). Our results showing that Hp does not induce significant alterations in the expression of any of the sialyltransferases or fucosyltransferases involved in sialyl-Lex biosynthesis suggest a mechanism by which an increased β3GnT5 expression may lead to the increased expression of sialyl-Lex antigen.
Figure 7. Schematic representation of the biosynthesis of Neo-lactoseries and terminal glycosylation.
The β3GnT5 is essential for the synthesis of type 2 chains on glycolipids. The glycan chain it generates, GlcNAc-LacCer, is a substrate for a β4Gal-T that forms the type 2 chain, which may be further substituted by sialyltransferases and fucosyltransferases completing the biosynthesis of various Lewis antigens, including the sialyl-Lex antigen. The action of other glycosyltransferases may also lead to termination of the chain with the formation of histo-blood groups H, A, and B. Alternatively, the type 2 chain may also be extended by the action of iGnT, leading to the biosynthesis polylactosamines, formation of polymeric Lewis antigens, and termination of the chain.
In addition, our observation showing a reduced expression of iGnT may lead to less extended carbohydrate chains, since this enzyme is essential for the extension of polylactosamines (27). These observations further support the hypothesis that the sialyl-Lex is synthesized on a rather short chain, which may facilitate proximity between Hp and the gastric epithelial cell.
To further clarify whether β3GnT5 upregulation was related to the degree of pathogenicity of Hp strains, we infected 2 different gastric cell lines, MKN45 and AGS, with a panel of 7 cagPAI+ and 5 cagPAI– Hp strains. Additionally, we used 2 isogenic mutants for genes present in the cagPAI, cagA and cagE, which encode essential components of the type IV secretion system. We measured IL8 gene induction as a reporter gene of the degree of pathogenicity of each Hp strain, since it is well established that an association exists between cagPAI status and IL-8 upregulation (28, 29). As expected, we observed that infection with cagPAI+ strains led to higher increases of IL8 gene expression in both cell lines, as opposed to cagPAI– strains, which induced minor increases (Figure 3, A and B). We also observed that infection with 60190ΔcagA significantly reduced IL-8 induction, whereas 60190ΔcagE showed no induction at all. This is in agreement with previous results in which cagA– mutants did not completely revert the induction of IL-8 but cagE- mutants maintained basal IL-8 expression levels (29–31).
We analyzed β3GnT5 expression levels and observed that only the cagPAI+ strains increased the expression of this glycosyltransferase, whereas cagPAI– strains showed no significant differences when compared with control cells without Hp (Figure 3, C and D). Interestingly, infection with 127UK strain led to no alteration of β3GnT5 or IL-8 expression levels, although 127UK has been characterized as cagPAI+ (32), leading us to speculate that it may be missing at least 1 essential gene within the PAI that impairs the induction of these genes. Similarly to what was observed with IL-8, mutant bacterial strains reduced β3GnT5 induction when compared with the isogenic 60190 parental strain. These results showed that cagA mutants were still able to stimulate β3GnT5 and IL-8 expression, although to a lesser extent than that of the parental strain, whereas cagE mutants were not. This suggests that β3GnT5 expression depends on both the translocating CagA molecule and the structural CagE, essential for a functional type IV secretion system (4, 33). In summary, β3GnT5 expression levels observed for each strain mimic those observed for IL-8 expression, thus confirming the hypothesis that β3GnT5 is a target for a very specific gene expression alteration induced solely by pathogenic Hp strains.
While our study focuses on the host cell responses to a pathogenic Hp infection, others have studied Hp gene expression alterations mediated by gastric cells. Kim et al. were able to demonstrate that direct adhesion to AGS cells leads to 43 altered Hp genes that may contribute to bacterial fitness. These genes affect outer membrane composition, translation of bacterial mRNA, and flagellar mediated motility (34). Recognition of flagellar proteins by TLR5 is a common mechanism of the innate immune system against pathogens. Interestingly, Hp flagella are fairly indifferent to TLR5 (35). Bidirectional interactions between Hp and gastric cells are therefore common and can benefit the bacteria and its chronic survival in the stomach. As Hp infection persists over a lifetime, it is mandatory that both the microbe and the host adapt to each other. Once chronicity is established, the immune stimulation appears remarkably constant, as antibody titers remain stable for over 20 years (36), consistent with a model of dynamic equilibrium (37).
Previous studies have shown that human bronchial mucosa explants exposed to TNF-α had an increased expression of several glycosyltransferases, namely fucosyltransferases and sialyltransferases (19). Studies on infected gastric mucosa cells have shown that there is a local enrichment of TNF-α in Hp-associated gastritis (38). This can result either from macrophage/lymphocyte production or as a consequence of direct interaction of Hp and gastric epithelial cells. We found TNF-α expression levels to be barely detectable in MKN45 cells alone but to dramatically increase after infection with low-Hp and to increase even more after infection with high-Hp (Figure 4A). TNF-α overexpression has also been observed after Hp infection in SNU-5 and KATO III gastric cell lines (39). In agreement with these observations, our results show the induction of genes downstream the TNF pathway, such as the small inducible cytokine A20 (CCL20) (40), GMCSF2 (38, 39), AP-1 component Jun-B, along with several genes of the NF-κB pathway: IL-8 (21–24), ICAM-1 (41), GRO1 (42) and syndecan-4 (43) (Table 1). In fact, genes involved in inflammation and in the activation of immune response displayed the most remarkable increases in response to Hp infection. In order to understand the effects of TNF-α in the regulation of β3GnT5 expression, we treated MKN45 cells with this proinflammatory cytokine and found that it led to increased expression of this enzyme (Figure 4C). Furthermore, our array experiments showed that the pathogenic Hp strain led to a 31.0-fold induction of TNFR9. This member of the TNF receptor superfamily, although being described as activated solely by its ligand 4-1BBL, has a similar downstream signaling as TNF-α, through the TRAF2-NIK pathway activating NF-κB (44), and leading to enhanced IL-8 production (45). On the other hand, although we observed that infection with pathogenic Hp strains led to IL-8 overexpression, this proinflammatory molecule was not capable of directly inducing β3GnT5 expression per se in our cell stimulation assays. This suggests that IL-8 and β3GnT5 may be downstream results of a common signaling pathway but that β3GnT5 is not directly dependent on IL-8 increase. Our observations therefore suggest a mechanism in which an Hp infection leads to overexpression of TNF-α and TNFR9, subsequently inducing several genes downstream the TNF pathway, including the glycosyltransferase β3GnT5.
In order to study the effects of an upregulation of this enzyme in the glycosylation profile of gastric cells, we stably transfected MKN45 and AGS cells with the full-length human β3GnT5 and analyzed the expression of the blood group Lewis antigens. For the synthesis of the different terminal Lewis structures, action of several glycosyltransferases is required, which determines the fate of the backbone chain, modulating the extension of the chain and the terminal structure formation with, for example, fucosylation and/or sialylation steps. The outcome of this process depends on the repertoire of glycosyltransferases that a given cell expresses at a particular time. To understand the effects of β3GnT5 overexpression in Hp ligand formation, namely sialyl-Lex synthesis, we used 2 different gastric cell line models, AGS, which is devoid of sialyl-Lex, and MKN45, which expresses moderate amounts of this antigen, implying an active pathway toward sialyl-Lex synthesis with the required fucosyl and sialyltransferase enzymatic activities. We observed that overexpression of β3GnT5 altered the glycosylation profiles of both MKN45 and AGS cells, with an upregulation of sialyl-Lex antigen expression (Table 2 and Figure 4). This was the only alteration common to both cell lines when compared with their mock-transfected and parental counterparts. MKN45 also showed an overexpression of dimeric sialyl-Lex, whereas AGS showed an increase of Lea, sialyl-Lea, and Leb. All other Lewis antigens remained unchanged. It is noteworthy that sialyl-Lex and sialyl-Lea have structural similarities that enable SabA to recognize and bind to both glycans (20). The variation of the glycosylation profile between cell lines depends on the glycosylation background of each cell line, which is the reflection of the repertoire of active glycosyltransferases. Mock-transfected MKN45 (MKN45mock) cells had a 25.2-fold higher expression of ST3Gal IV when compared with mock-transfected AGS (AGSmock) cells (Supplemental Figure 2). This higher baseline may explain why MKN45 expresses some sialyl-Lex, whereas AGS lacks expression. This may be the reason why overexpression of β3GnT5 in AGS-T5 cells leads to lower sialyl-Lex expression compared with MKN-T5 cells, which is in line with the strong staining for Ley observed for virtually all AGS cells (Table 2). Reduced sialylation of the Gal residue of the backbone type 2 chain may favor Ley synthesis, to the detriment of sialyl-Lex synthesis. Therefore, even with the overexpression of β3GnT5 and enhanced synthesis of type 1 and 2 backbone chains, there is little sialyl-Lex formation in AGS cells, in contrast to MKN45 cells, which express Ley in less than 25% of cells (Table 2). Whether β3GnT5 upregulation is also involved in other pathological conditions that involve aberrant expression of sialyl-Lex antigen remains to be clarified.
In order to study the effect of β3GnT5 in Hp pathogenesis, we evaluated the ability of Hp to adhere to gastric cells in the context of β3GnT5 overexpression and subsequent sialyl-Lex synthesis. Our findings showed that Hp strains J99 and babA1A2-mutant, both having an active SabA, significantly increased their binding towards the MKN-T5 cells when compared with MKN45wt cells (Figure 6). The babA1A2-mutant is derived from the same parental strain as 17875/Leb, and these 2 strains have opposite binding capabilities: the first lacks BabA adhesin and therefore binds only to sialyl-Lex antigen; the latter is incapable of binding to sialyl-Lex and binds only to Leb (20). Strikingly, 17875/Leb strain showed no differences in adhesion in MKN45wt compared with MKN-T5 cells. These results are in line with the fact that transfection of β3GnT5 led to no alterations in carbohydrate ligands other than sialyl-Lex antigen. Increased epithelial adherence should benefit Hp by providing access to nutrient-rich tissues. On the other hand, such proximity and consistency of attachment would lead to a stronger host immune response, accompanied by intense inflammation. This suggests that the lifelong persistence of Hp greatly depends on an equilibrium between effective attachment and plasticity of the binding properties of bacterial adhesins. SabA is fundamental for the close binding to the gastric cells (14), which contributes to the establishment of the type IV secretion system. This adhesin is also known to rapidly adapt to the glycosylation profiles that dynamically change in inflammation states by an on/off expression mechanism and a polymorphic binding nature (14, 20). Controlling the expression of sialyl-Lex by β3GnT5 overexpression at certain stages of infection may be yet another important mechanism by which Hp adapts to the surrounding milieu and maintains this lifelong equilibrium. Our results demonstrate the impact of β3GnT5 and sialyl-Lex in Hp-host interaction, unravelling their role in Hp pathogenesis. Studies using in vivo models will further clarify the relevance of this glycosylation pathway in pathogenesis associated with cagPAI+ Hp strains.
In summary, we conclude the following: (a) Hp induces significant alterations in mRNA expression of a small fraction of the 1,031 human genes tested. (b) All genes altered by low-pathogenic strain were consistently altered to a higher fold extent by a high-pathogenic strain, reinforcing the role of the virulence factors of each strain in the host gene expression. (c) Genes involved in the regulation of the inflammatory response reproduce in vivo data and contribute to the understanding of the typical neutrophilic and lymphocytic infiltration characteristic of Hp infection. (d) There is an increased expression of β3GnT5, a GlcNAc transferase essential for the biosynthesis of Lewis antigens, and this induction is specific to pathogenic Hp strains (cagPAI+). This induction is dependent of cagA and cagE, most probably through the TNF/NF-κB pathway. (e) Overexpression of β3GnT5 leads to sialyl-Lex formation, indicating a mechanism by which Hp modulates the synthesis of the SabA ligand. (f) This mechanism promotes increased Hp adhesion to gastric cells, demonstrating the impact of this molecular mechanism in gastric pathogenesis.
Methods
Bacteria and gastric cell lines.
Human gastric carcinoma cell lines MKN45 (obtained from the Japanese Collection of Research Bioresources) and AGS (obtained from ATCC) were grown in RPMI 1640 medium with Glutamax (Invitrogen) supplemented with 10% inactive fetal bovine serum (Invitrogen), 50 μg/ml gentamicin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2.
Hp cagPAI+ strains 101UK, 127UK, BO265, 111UK, and CM71 and cagPAI– strains 094UK, 097UK, 131UK, and 123UK were obtained from the Max-Planck Institut für Infektionsbiology, Berlin, Germany (32, 46). vacA s1/m1, cagPAI+ strain 26695 (ATCC 700392), vacA s2/m2, cagPAI– strain Tx30a (ATCC 51932), cagPAI+ strain 60190 (ATCC 49503), and cagPAI+ J99 (ATCC 700824) were obtained from ATCC. Hp strain 60190 insertion mutants with inactivation of cagA (60190ΔcagA) or cagE (60190ΔcagE) were obtained from the Institute of Infection, Immunity and Inflammation, University of Nottingham, United Kingdom. The Hp strains 17875/Leb, a spontaneous variant unable to bind sialyl-Lex isolated from strain 17875, and 17875babA1:kan babA2:cam (isogenic mutant of 17875 strain, abbreviated babA1A2-mutant) were obtained from Umeå University, Umeå, Sweden (14).
All bacteria strains were grown in tryptic soy agar supplemented with 5% sheep’s blood (BioMérieux, Marcy l’ωtoile, France) at 37°C under microaerobic conditions. For strain babA1A2-mutant, culture media also included 20 mg/l chloramphenicol (Sigma-Aldrich) and 25 mg/l kanamycin (Sigma-Aldrich).
Infection procedures.
Hp strains were grown for 3 days as described above, after which they were collected and resuspended in Brucella broth (Becton Dickinson). MKN45 and AGS cells were washed and seeded at a density of 3.0 × 106 cells/T75 24 h prior to infection, in regular medium without antibiotics. Bacteria were added to fresh RPMI 1640 medium supplemented with 10% FBS and incubated with MKN45 and AGS cells for 8 h at an MOI of 100. Control experiments were performed using sterile Brucella broth.
Infection experiments intended for subsequent microarray hybridization were performed with MKN45 infected with high-Hp and low-Hp. For each biological condition (without Hp, with high-Hp, or with low-Hp), 3 independent replicate experiments were performed in triplicate. From these triplicates, total RNA was extracted, and a pooling strategy was used. This strategy is recommended in cell culture experiments that involve the use of 3 independent biological conditions, resulting in an increase of the statistical power of the data collected (47).
RNA extraction and analysis of mRNA expression by RT-PCR and real-time PCR.
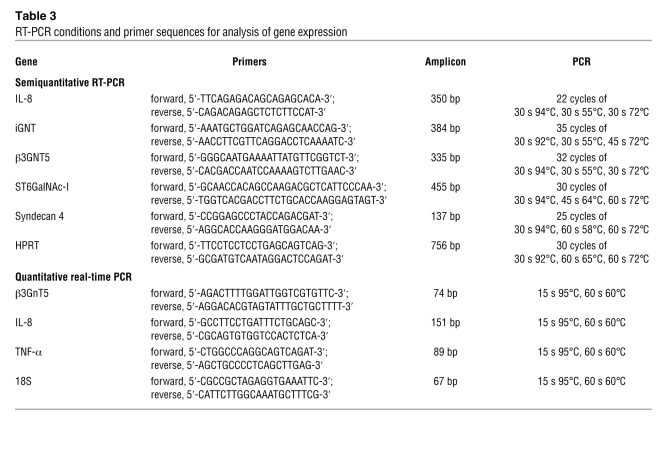
Total RNA was extracted using the RNeasy isolation kit (QIAGEN) according to the manufacturer’s protocol, which involves a final step with a cleanup column to wash all traces of solvents from the RNA. RNA yield and quality were determined spectrophotometrically, and 5.0 μg of total RNA was reverse transcribed using the Superscript III RNase H Reverse Transcriptase kit (Invitrogen) according to the manufacturer’s instructions. In order to control the yield of cDNA synthesis as well as any genomic DNA contamination, a PCR with primers in different exons, for amplification of the hypoxanthine phosphoribosyltransferase (HPRT) mRNA, was performed. HPRT, IL-8, β3GNT5, iGnT, ST6GalNAc-I, and Syndecan-4 were amplified as described in Table 3.
Table 3 .
RT-PCR conditions and primer sequences for analysis of gene expression
PCR reaction mixture contained 1 μl cDNA, 5.0 μl PCR buffer (500 mM KCl, 15 mM MgCl2, 100 mM Tris-HCl, pH 9.0), 3.0 μl 10 mM dNTPs, 0.4 μl Taq DNA polymerase, 4.0 μl of each 3-mM primer, and H2O to a total volume of 25 μl. Reaction products obtained were submitted to electrophoresis in 1.7% agarose gels containing ethidium bromide, and bands were quantified using Quantity One software (BioRad).
Quantitative analysis of β3GnT5, IL-8, and TNF-α expression was performed using real-time PCR using 10.0 μl SYBRgreen reagent (Applied Biosystems), 0.6 μl of each 10-mM primer (Table 3), and 4 μl 1:100 diluted cDNA. Each sample was amplified in triplicate in an ABI Prism 7000 (Applied Biosystems). Expression of 18S was also measured in triplicate for each sample and used for normalization of target gene abundance. Specificity of amplification was confirmed by melt curve analysis. Standard curve was determined for each gene, and results are presented as a ratio between target gene and 18S quantity.
Microarray procedures and analysis of data.
Three independent infection experiments were performed with each Hp strain as well as without Hp. RNA from triplicate preparations was labeled using standard Affymetrix protocols for 3′ expression arrays (48). A total of 9 samples were hybridized to the Consortium for Functional Glycomics (CFG) GLYCOv2 glycogene array. All arrays were scanned using the Affymetrix Scanner 3000. The GLYCOv2 Gene Chip array contains probe sets to monitor the expression of the 1,031 human transcripts and was developed by the CFG using Affymetrix technology. The following classes of genes are represented on the GLYCOv2 Gene Chip: glycosyltransferases, glycan-binding proteins, sulfotransferases, C-type lectins, neuraminidases, nucleotide sugar synthesis and transporter proteins, N-glycan biosynthesis–related genes, mucins, proteoglycans, growth factors, cytokines, chemokines, conserved oligomeric Golgi complex genes, and others. A full list of the genes monitored by the GLYCOv2 Gene Chip array is available at http://www.functionalglycomics.org/static/consortium/resources/resourcecoree.shtml.
On the GLYCOv2, transcripts were detected using either 2 (for non-glyco genes) or 3 (for glyco-genes) identical probe sets. Each gene probe set consisted of eleven 25-mer oligonucleotide “perfect match” probes and eleven 25-mer “mismatch” probes with a single base mismatch at the center position. Detection calls were calculated using the GeneChip Operating Software (GCOS; Affymetrix) algorithm, in which the hybridization intensities from each 25-mer perfect match probe are compared with those of the associated mismatched probe. GCOS uses a Wilcoxon signed-rank test to identify significant differences among the perfect match–mismatch probe pairs in a probe set, assigning a “present” call for probe sets with P values less than 0.04.
Expression signal values were generated using the RMA algorithm (49). Quantile normalization and background subtraction were then performed to generate base 2 log-transformed expression values for all probe sets on the GLYCOv2. Only genes called as “present” in at least 2 of the 3 arrays performed for any 1 experimental condition were used in further analyses. Using BRB-ArrayTools 3.2.2 software ( http://linus.nci.nih.gov/BRB-ArrayTools.html), replicate probe set copies on each array were averaged and gene expression patterns were analyzed using hierarchical clustering and class comparison methods.
ANOVA was performed using all samples from the 3 groups. Based on this data, a hierarchical clustering was performed using centered correlation and average linkage. Class comparisons were performed using a univariate cut-off of 0.001 and a multivariate permutation-based false discovery rate calculation. The false discovery rate calculation was set at a confidence level of 80%, and the cut-off for the predicted proportion of false discoveries was set at 10%. Combining these 2 criteria, 3 class comparisons were made (high-Hp vs. without Hp, low-Hp vs. without Hp, and high-Hp vs. low-Hp), which rendered lists of 168, 49, and 79 genes, respectively.
The entire microarray data set and data analysis is publicly available in the CFG gateway ( http://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp; investigator: Celso A. Reis).
Cytokine stimulation assays.
For TNF-α stimulation assays, 1.5 × 106 cells were grown in a T25 flask for 24 h in regular medium. Cells were then maintained in the absence (control) or presence of 40 ng/ml TNF-α (PeproTech Inc.) for 24 h, 48 h, and 72 h.
For IL-8 stimulation assays, 1.0 × 106 cells were grown in 60-mm culture dishes for 24 h in regular medium. Cells were then maintained in the absence (control) or presence of 50 ng/ml IL-8 (Sigma-Aldrich) for 8 h and 24 h.
Human β3GnT5 transfection and characterization of transfectants.
Human full-length β3GnT5 was a kind gift from H. Narimatsu (Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan). Complete ORF was cloned, inserted into pcDNA3.1 plasmid, and fully sequenced. AGS and MKN45 cells were stably transfected with β3GnT5full-pcDNA3.1 or empty vector using the Lipofectamine 2000 reagent at a 3:1 ratio to DNA and the Plus reagent in OPTI-MEM medium (all Invitrogen) following the manufacturer’s instructions. Selection was initiated after 48 h in regular medium supplemented with 0.6 mg/ml G418 (Invitrogen). Cells were maintained under selection for 2–3 wk, after which G418 concentration was changed to 0.3 mg/ml in regular medium. Transfected cells were renamed MKN-T5 and AGS-T5.
Characterization of glycosylation was performed by immunofluorescence. Procedures and mAb references have been previously described (50). mAb anti–sialyl-Lex, clone KM93, was purchased from Chemicon International and used according to the same protocol at a 1:40 dilution.
Hp adhesion assays.
Hp was labeled with FITC, and the concentration was adjusted as previously described (51). Cell lines MKN45, MKN45neo, and MKN-T5 were cultured in multi-well slides until near confluence. Adhesion assays were performed as previously described (51). Briefly, cells were fixed with cold acetone for 5 min, followed by incubation for 1 h with blocking buffer: 1% BSA in PBS containing 0.05% Tween-20. BSA was previously submitted to periodate oxidation to destroy competitive carbohydrate receptors for Hp binding (20).The FITC-labeled bacterial suspension was diluted 5-fold in blocking buffer, and 100 μl was placed on each slide well, followed by incubation for 2 h at room temperature. Slides were subsequently washed 4 times with PBS containing 0.05% Tween-20, stained with DAPI, and again washed 4 times.
Evaluation of adhesion was performed after acquisition of 5 different fields under ×400 magnification. Quantification of bacterial adhesion was obtained with the ImageJ software, and significance was evaluated using the Student’s t test.
Supplementary Material
Acknowledgments
We kindly thank M. Achtman from the Max-Planck Institut für Infektionsbiology, Thomas Borén from Umeå University, Sara Lindén from the University of Queensland, Rachel Thomas, Richard Argent, and John C. Atherton from the Institute of Infection, Immunity and Inflammation, University of Nottinghamfor providing the Hp strains. We kindly thank H. Narimatsu and A. Togayachi for the plasmid containing the human β3GnT5. We kindly thank Paula Sampaio for assistance in fluorescence quantification. This work was supported by Fundação para a Ciência e a Tecnologia (grants POCI/SAU-OBS/56686/2004, PTDC/SAU-MII/64153/2006, and POCI/SAU-IMI/56681/2004), Programa Operacional Ciência e Inovação 2010 do Quadro Comunitário de Apoio III e comparticipado pelo Fundo Europeu de Desenvolvimento Regional; and Association for International Cancer Research (grant 05-088). Grants were received from the Fundação para a Ciência e a Tecnologia (SFRH/BD/11764/2003, to N.T. Marcos and SFRH/BD/36339/2007, to A. Magalhães). N.T. Marcos acknowledges the GABBA Program of the University of Porto. The resources and collaborative efforts provided by the Consortium for Functional Glycomics were funded by grant NIGMS-GM62116.
Footnotes
Nonstandard abbreviations used: β3GnT5, β3 GlcNAc T5; cagPAI, cag pathogenicity island; high-Hp, high-pathogenicity 26695 Hp strain; Hp, Helicobacter pylori; iGnT, I β3 GlcNAc T; Lex, Lewis x; low-Hp, low-pathogenicity Tx30a Hp strain; SabA, sialic acid–binding adhesin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2325–2336 (2008). doi:10.1172/JCI34324
References
- 1. International Agency for Research on Cancer. 2003.World cancer report. IARC Press. Lyon, France. 352 pp. [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process — First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.Parsonnet J., et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S., Michetti P. Helicobacter pylori infection. N. Engl. J. Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Wang C.C., et al. Helicobacter pylori infection and age on the development of intestinal metaplasia--a multiple logistic regression analysis. Hepatogastroenterology. 1998;45:2234–2237. [PubMed] [Google Scholar]
- 6.Fujioka T., Honda S., Tokieda M. Helicobacter pylori infection and gastric carcinoma in animal models. J. Gastroenterol. Hepatol. 2000;15(Suppl.):D55–D59. doi: 10.1046/j.1440-1746.2000.02139.x. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/S0016-5085(98)70143-X. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Q., Chen X.Y., Shi Y., Xiao S.D. Development of gastric adenocarcinoma in Mongolian gerbils after long-term infection with Helicobacter pylori. J. Gastroenterol. Hepatol. 2004;19:1192–1198. doi: 10.1111/j.1440-1746.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 9.Cover T.L., Blaser M.J. Helicobacter pylori factors associated with disease. Gastroenterology. 1999;117:257–261. doi: 10.1016/S0016-5085(99)70575-5. [DOI] [PubMed] [Google Scholar]
- 10.Peek R.M., Jr., Blaser M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard M., et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Doorn L.J., et al. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/S0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 13.Ilver D., et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 14.Mahdavi J., et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson J.C., Colley K.J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J. Biol. Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 16.Holmes E.H., Hakomori S., Ostrander G.K. Synthesis of type 1 and 2 lacto series glycolipid antigens in human colonic adenocarcinoma and derived cell lines is due to activation of a normally unexpressed beta 1-3N-acetylglucosaminyltransferase. J. Biol. Chem. 1987;262:15649–15658. [PubMed] [Google Scholar]
- 17.Holmes E.H., Ostrander G.K., Clausen H., Graem N. Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. Regulation through type 2 core chain synthesis rather than fucosylation. J. Biol. Chem. 1987;262:11331–11338. [PubMed] [Google Scholar]
- 18.Togayachi A., et al. Molecular cloning and characterization of UDP-GlcNAc:lactosylceramide beta 1,3-N-acetylglucosaminyltransferase (beta 3Gn-T5), an essential enzyme for the expression of HNK-1 and Lewis X epitopes on glycolipids. J. Biol. Chem. 2001;276:22032–22040. doi: 10.1074/jbc.M011369200. [DOI] [PubMed] [Google Scholar]
- 19.Delmotte P., et al. Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J. Biol. Chem. 2002;277:424–431. doi: 10.1074/jbc.M109958200. [DOI] [PubMed] [Google Scholar]
- 20.Aspholm M., et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS. Pathog. 2006;2:e110. doi: 10.1371/journal.ppat.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aihara M., et al. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect. Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glocker E., et al. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-kappaB activation. Infect. Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebkova L., et al. Extracellular signal-regulated protein kinase mediates interleukin 17 (IL-17)-induced IL-8 secretion in Helicobacter pylori-infected human gastric epithelial cells. Infect. Immun. 2004;72:5019–5026. doi: 10.1128/IAI.72.9.5019-5026.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S.A., Tummuru M.K., Blaser M.J., Kerr L.D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 25.Marcos N.T., et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 26.Harduin-Lepers A., et al. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/S0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 27.van den Eijnden D.H., Winterwerp H., Smeeman P., Schiphorst W.E. Novikoff ascites tumor cells contain N-acetyllactosaminide beta 1 leads to 3 and beta 1 leads to 6 N-acetylglucosaminyltransferase activity. J. Biol. Chem. 1983;258:3435–3437. [PubMed] [Google Scholar]
- 28.Kim S.Y., Lee Y.C., Kim H.K., Blaser M.J. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2006;8:97–106. doi: 10.1111/j.1462-5822.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S.A., Tummuru M.K., Miller G.G., Blaser M.J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh J.T., Torres V.J., Cover T.L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 31.Maeda S., et al. cDNA microarray analysis of Helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem. Biophys. Res. Commun. 2001;284:443–449. doi: 10.1006/bbrc.2001.5006. [DOI] [PubMed] [Google Scholar]
- 32.Falush D., et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 33.Odenbreit S., et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 34.Kim N., et al. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect. Immun. 2004;72:2358–2368. doi: 10.1128/IAI.72.4.2358-2368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gewirtz A.T., et al. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 2004;189:1914–1920. doi: 10.1086/386289. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Perez G.I., et al. Evidence that cagA(+) Helicobacter pylori strains are disappearing more rapidly than cagA(–) strains. Gut. 2002;50:295–298. doi: 10.1136/gut.50.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaser M.J., Atherton J.C. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller A., Merrell D.S., Grimm J., Falkow S. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology. 2004;127:1446–1462. doi: 10.1053/j.gastro.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 39.Jung H.C., Kim J.M., Song I.S., Kim C.Y. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J. Gastroenterol. Hepatol. 1997;12:473–480. doi: 10.1111/j.1440-1746.1997.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y.Y., et al. Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect. Immun. 2007;75:4357–4363. doi: 10.1128/IAI.01660-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori N., et al. Induction of monocyte chemoattractant protein 1 by Helicobacter pylori involves NF-kappaB. Infect. Immun. 2001;69:1280–1286. doi: 10.1128/IAI.69.3.1280-1286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Eck M., et al. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin. Exp. Immunol. 2000;122:192–199. doi: 10.1046/j.1365-2249.2000.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith M.F., Jr., Novotny J., Carl V.S., Comeau L.D. Helicobacter pylori and toll-like receptor agonists induce syndecan-4 expression in an NF-{kappa}B-dependent manner. Glycobiology. 2006;16:221–229. doi: 10.1093/glycob/cwj061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinay D.S., Kwon B.S. Role of 4-1BB in immune responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 45.Salih H.R., et al. Constitutive expression of functional 4-1BB (CD137) ligand on carcinoma cells. J. Immunol. 2000;165:2903–2910. doi: 10.4049/jimmunol.165.5.2903. [DOI] [PubMed] [Google Scholar]
- 46.Suerbaum S., et al. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng X., et al. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics. 2003;4:26. doi: 10.1186/1471-2105-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lockhart D.J., et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 49.Irizarry R.A., et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinho S., et al. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2006;249:157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Falk P., et al. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.