Abstract
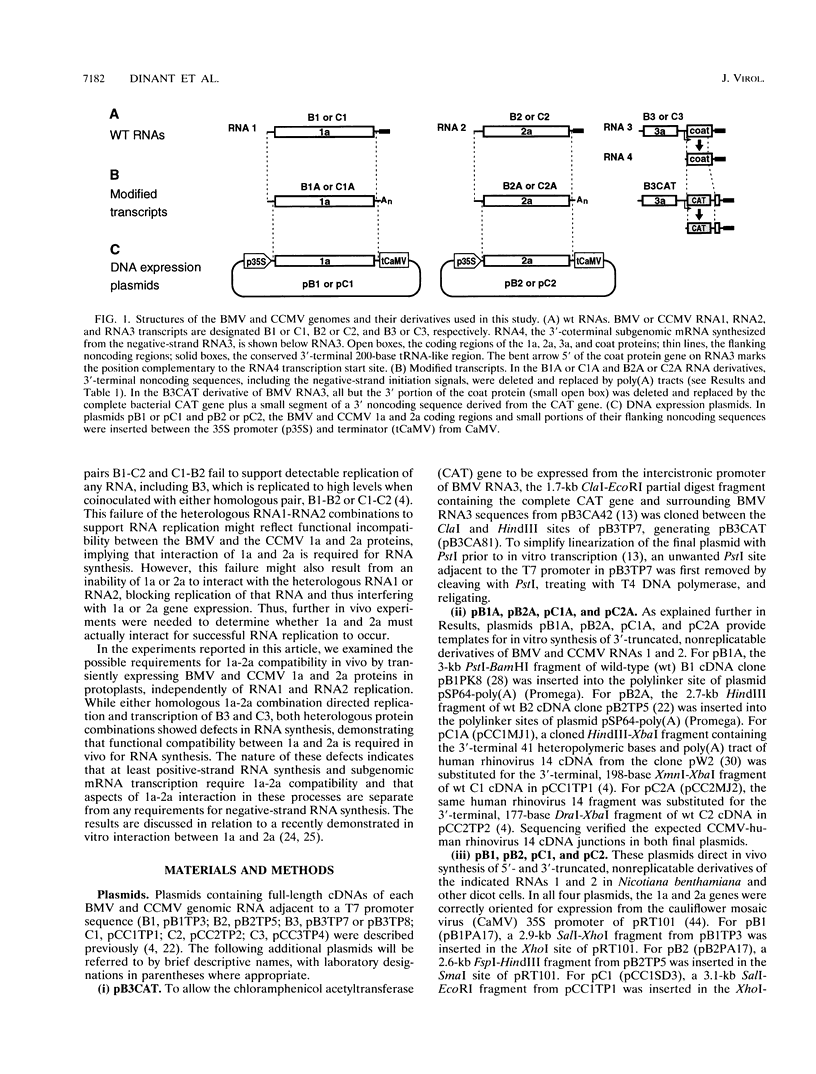
The positive-strand RNA bromoviruses encode two nonstructural proteins, 1a and 2a, involved in RNA-dependent RNA replication. These proteins have extensive sequence similarities with methyltransferase, helicase, and polymerase proteins of other plant and animal viruses. 1a and 2a can also form a complex in vitro. To explore whether 1a-2a interaction is required for RNA replication in vivo, we reassorted the 1a and 2a genes from two different bromoviruses, brome mosaic virus (BMV) and cowpea chlorotic mottle virus (CCMV). 1a and 2a were expressed independently of viral replication by using RNA- or DNA-based transient expression, and their in vivo RNA replication activities were tested in protoplasts with BMV and CCMV RNA3 templates. RNA-based transient expression confirmed prior indications that bromovirus RNA replication is more sensitive to reductions in 1a expression than to reductions in 2a expression. DNA-based expression of the homologous combinations of 1a and 2a supported high levels of RNA synthesis, but both 1a-2a heterologous combinations exhibited RNA synthesis defects. The combination of CCMV 1a and BMV 2a did not support detectable synthesis of negative-strand, positive-strand, or subgenomic RNA. The converse combination of BMV 1a and CCMV 2a was preferentially defective in positive-strand and subgenomic RNA accumulation, showing that 1a-2a interaction is involved in these processes in ways distinct from negative-strand RNA synthesis, which was only slightly affected. These results indicate that at least some functions of 1a and 2a operate in a mutually dependent manner in vivo and that the mechanisms of positive- and negative-strand RNA synthesis are differentiated in part by features of such interactions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P. Bromovirus RNA replication and transcription. Curr Opin Genet Dev. 1992 Feb;2(1):71–76. doi: 10.1016/s0959-437x(05)80325-9. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R. F., Janda M., Ahlquist P. Infectious in vitro transcripts from cowpea chlorotic mottle virus cDNA clones and exchange of individual RNA components with brome mosaic virus. J Virol. 1988 Oct;62(10):3581–3588. doi: 10.1128/jvi.62.10.3581-3588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer C. W., Kaper J. M. Double-stranded RNAs of cucumber mosaic virus and its satellite contain an unpaired terminal guanosine: implications for replication. Virology. 1985 Sep;145(2):249–259. doi: 10.1016/0042-6822(85)90158-8. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Zaitlin M. Capping of tobacco mosaic virus RNA. Analysis of viral-coded guanylyltransferase-like activity. J Biol Chem. 1990 May 15;265(14):7779–7786. [PubMed] [Google Scholar]
- French R., Ahlquist P. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J Virol. 1988 Jul;62(7):2411–2420. doi: 10.1128/jvi.62.7.2411-2420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Walbot V. RNA pseudoknot domain of tobacco mosaic virus can functionally substitute for a poly(A) tail in plant and animal cells. Genes Dev. 1990 Jul;4(7):1149–1157. doi: 10.1101/gad.4.7.1149. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988 Aug 1;235(1-2):16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Motoyoshi F., Takamatsu N., Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986 Nov 11;14(21):8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M., Ahlquist P. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell. 1993 Mar 26;72(6):961–970. doi: 10.1016/0092-8674(93)90584-d. [DOI] [PubMed] [Google Scholar]
- Janda M., French R., Ahlquist P. High efficiency T7 polymerase synthesis of infectious RNA from cloned brome mosaic virus cdna and effects of 5' extensions on transcript infectivity. Virology. 1987 May;158(1):259–262. doi: 10.1016/0042-6822(87)90265-0. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Ahlquist P. Identification of the domains required for direct interaction of the helicase-like and polymerase-like RNA replication proteins of brome mosaic virus. J Virol. 1992 Dec;66(12):7293–7302. doi: 10.1128/jvi.66.12.7293-7302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Quadt R., Hershberger R. P., Ahlquist P. Brome mosaic virus RNA replication proteins 1a and 2a from a complex in vitro. J Virol. 1992 Nov;66(11):6322–6329. doi: 10.1128/jvi.66.11.6322-6329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner P. A., Young B. M., Ahlquist P. Analysis of the role of brome mosaic virus 1a protein domains in RNA replication, using linker insertion mutagenesis. J Virol. 1990 Dec;64(12):6110–6120. doi: 10.1128/jvi.64.12.6110-6120.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner P., Richards D., Traynor P., Ahlquist P. Defined mutations in a small region of the brome mosaic virus 2 gene cause diverse temperature-sensitive RNA replication phenotypes. J Virol. 1989 Dec;63(12):5302–5309. doi: 10.1128/jvi.63.12.5302-5309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L. E., Huntley C. C., Pogue G. P., Connell J. P., Hall T. C. Regulation of (+):(-)-strand asymmetry in replication of brome mosaic virus RNA. Virology. 1991 May;182(1):76–83. doi: 10.1016/0042-6822(91)90650-z. [DOI] [PubMed] [Google Scholar]
- Mi S., Stollar V. Expression of Sindbis virus nsP1 and methyltransferase activity in Escherichia coli. Virology. 1991 Sep;184(1):423–427. doi: 10.1016/0042-6822(91)90862-6. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Bujarski J. J., Dreher T. W., Hall T. C. Minus-strand initiation by brome mosaic virus replicase within the 3' tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986 Feb 20;187(4):537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Mise K., Allison R. F., Janda M., Ahlquist P. Bromovirus movement protein genes play a crucial role in host specificity. J Virol. 1993 May;67(5):2815–2823. doi: 10.1128/jvi.67.5.2815-2823.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha R. F., Allison R. F., Ahlquist P. cis-acting sequences required for in vivo amplification of genomic RNA3 are organized differently in related bromoviruses. Virology. 1990 Feb;174(2):436–443. doi: 10.1016/0042-6822(90)90097-b. [DOI] [PubMed] [Google Scholar]
- Pogue G. P., Marsh L. E., Connell J. P., Hall T. C. Requirement for ICR-like sequences in the replication of brome mosaic virus genomic RNA. Virology. 1992 Jun;188(2):742–753. doi: 10.1016/0042-6822(92)90529-x. [DOI] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Purification and characterization of brome mosaic virus RNA-dependent RNA polymerase. Virology. 1990 Sep;178(1):189–194. doi: 10.1016/0042-6822(90)90393-6. [DOI] [PubMed] [Google Scholar]
- Quadt R., Kao C. C., Browning K. S., Hershberger R. P., Ahlquist P. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1498–1502. doi: 10.1073/pnas.90.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Requirement for a viral trans-acting factor encoded by brome mosaic virus RNA-2 provides strong selection in vivo for functional recombinants. J Virol. 1990 May;64(5):2437–2441. doi: 10.1128/jvi.64.5.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Huntley C. C., Marsh L. E., Hall T. C. Analysis of RNA stability and (-) strand content in viral infections using biotinylated RNA probes. J Virol Methods. 1990 Dec;30(3):239–250. doi: 10.1016/0166-0934(90)90066-o. [DOI] [PubMed] [Google Scholar]
- Rozanov M. N., Koonin E. V., Gorbalenya A. E. Conservation of the putative methyltransferase domain: a hallmark of the 'Sindbis-like' supergroup of positive-strand RNA viruses. J Gen Virol. 1992 Aug;73(Pt 8):2129–2134. doi: 10.1099/0022-1317-73-8-2129. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Gomatos P. J. Replication of semliki forest virus: polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J Virol. 1976 Nov;20(2):446–464. doi: 10.1128/jvi.20.2.446-464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidel L. M., Stollar V. Mutations that confer resistance to mycophenolic acid and ribavirin on Sindbis virus map to the nonstructural protein nsP1. Virology. 1991 Apr;181(2):490–499. doi: 10.1016/0042-6822(91)90881-b. [DOI] [PubMed] [Google Scholar]
- Traynor P., Ahlquist P. Use of bromovirus RNA2 hybrids to map cis- and trans-acting functions in a conserved RNA replication gene. J Virol. 1990 Jan;64(1):69–77. doi: 10.1128/jvi.64.1.69-77.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor P., Young B. M., Ahlquist P. Deletion analysis of brome mosaic virus 2a protein: effects on RNA replication and systemic spread. J Virol. 1991 Jun;65(6):2807–2815. doi: 10.1128/jvi.65.6.2807-2815.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R., Matzeit V., Gronenborn B., Schell J., Steinbiss H. H. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987 Jul 24;15(14):5890–5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]