Abstract
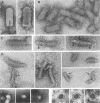
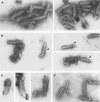
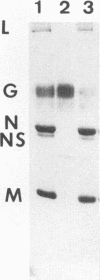
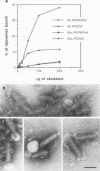
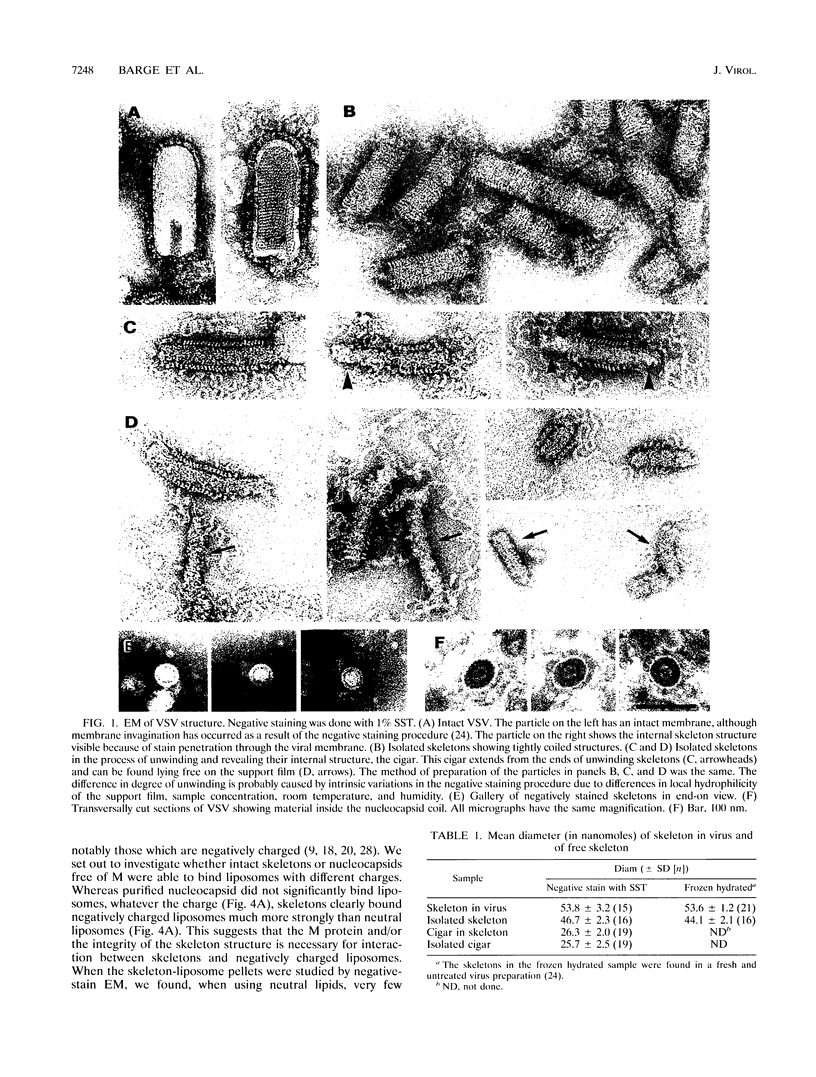
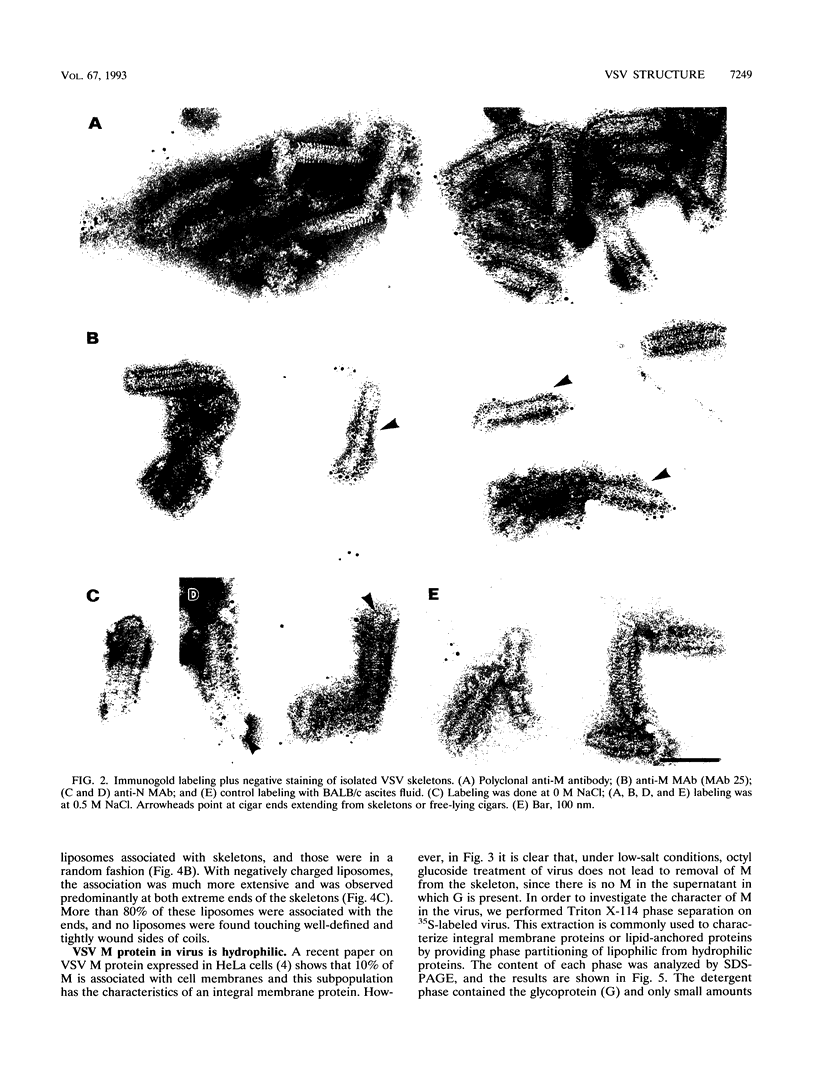
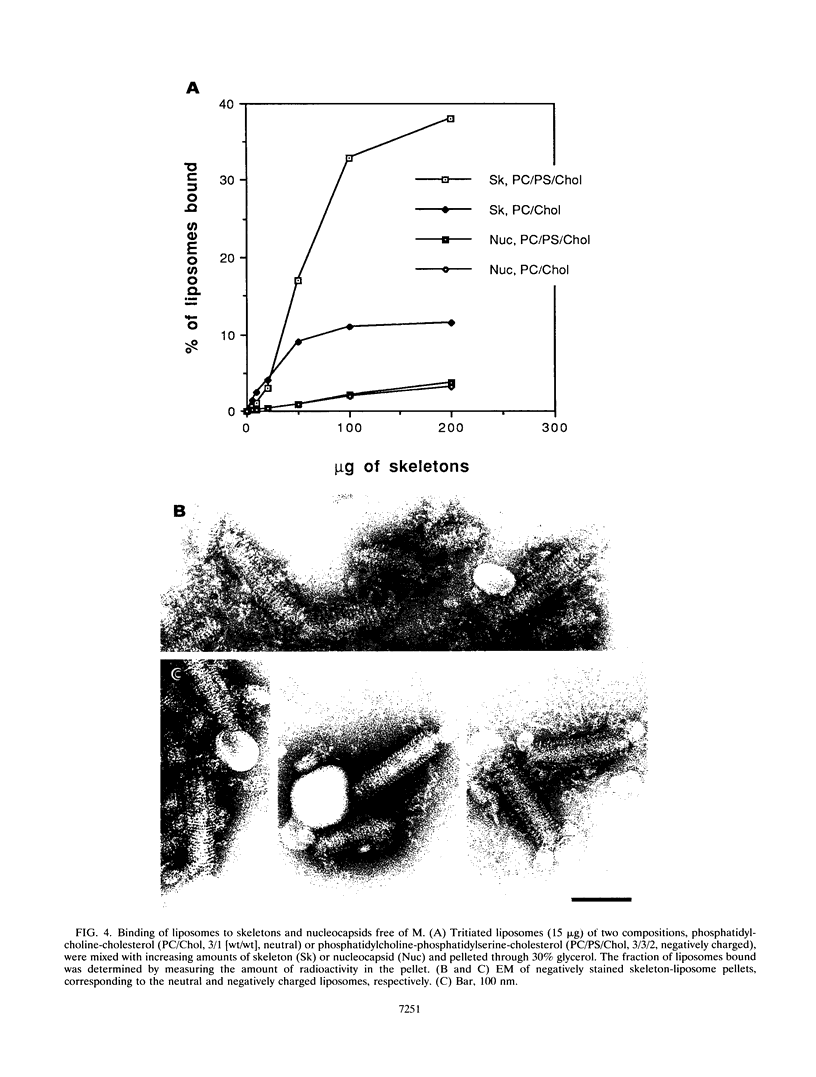
Vesicular stomatitis virus is an enveloped virus with an external glycoprotein G and a nucleocapsid that form, together with the M protein, a tight helically coiled structure: the skeleton. Negative staining and immunoelectron microscopy studies on skeleton preparations were performed to determine the localization of the M protein. These studies have resulted in a new model for the structure of rhabdoviruses in which the nucleocapsid is wound around a core containing the M protein. This model predicts contact between M and lipid only at the extreme ends of the skeleton, which is confirmed by skeleton-liposome binding studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L. D., Rose J. K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993 Jan;67(1):407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Raux H., Gaudin Y., Ruigrok R. W. Mechanisms of rabies virus neutralization. Virology. 1993 May;194(1):302–313. doi: 10.1006/viro.1993.1261. [DOI] [PubMed] [Google Scholar]
- Gaudin Y., Tuffereau C., Benmansour A., Flamand A. Fatty acylation of rabies virus proteins. Virology. 1991 Sep;184(1):441–444. doi: 10.1016/0042-6822(91)90866-a. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Vanderoef R. Localization of the membrane-associated region of vesicular stomatitis virus M protein at the N terminus, using the hydrophobic, photoreactive probe 125I-TID. J Virol. 1990 Jul;64(7):3486–3491. doi: 10.1128/jvi.64.7.3486-3491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Heterogeneity of vesicular stomatitis virus particles: implications for virion assembly. J Virol. 1980 Jan;33(1):52–58. doi: 10.1128/jvi.33.1.52-58.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy B. J., Jr, McKinnon K. P., Lyles D. S. Solubility of vesicular stomatitis virus M protein in the cytosol of infected cells or isolated from virions. J Virol. 1990 Feb;64(2):902–906. doi: 10.1128/jvi.64.2.902-906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Swanson R. E. In situ cross-linking of vesicular stomatitis virus proteins with reversible agents. Virology. 1978 Jul 15;88(2):263–280. doi: 10.1016/0042-6822(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Barenholz Y., Wagner R. R. Vesicular stomatitis virus membrane proteins and their interactions with lipid bilayers. Biochim Biophys Acta. 1987 Jun 24;906(2):175–193. doi: 10.1016/0304-4157(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Rayssiguier C., Cioe L., Withers E., Wunner W. H., Curtis P. J. Cloning of rabies virus matrix protein mRNA and determination of its amino acid sequence. Virus Res. 1986 Aug;5(2-3):177–190. doi: 10.1016/0168-1702(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doolittle R. F., Anilionis A., Curtis P. J., Wunner W. H. Homology between the glycoproteins of vesicular stomatitis virus and rabies virus. J Virol. 1982 Jul;43(1):361–364. doi: 10.1128/jvi.43.1.361-364.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R. W., Calder L. J., Wharton S. A. Electron microscopy of the influenza virus submembranal structure. Virology. 1989 Nov;173(1):311–316. doi: 10.1016/0042-6822(89)90248-1. [DOI] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvonarjev A. Y., Ghendon Y. Z. Influence of membrane (M) protein on influenza A virus virion transcriptase activity in vitro and its susceptibility to rimantadine. J Virol. 1980 Feb;33(2):583–586. doi: 10.1128/jvi.33.2.583-586.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]