Abstract
A low molecular weight, heat-resistant hepatotrophic factor in an extract from the bovine intestinal mucosa was purified and identified as ethanolamine by structural analyses. The mode of action of ethanolamine in vitro and in vivo coincided with that of the crude extract of the tissue, indicating that ethanolamine is the active component. Ethanolamine synergistically elevated the stimulation of DNA synthesis in hepatocytes in primary culture when added together with a growth factor, such as epidermal growth factor, with the ED50 being 20 μM, although it showed little stimulatory effect by itself. Contrary to these in vitro results, the intraperitoneal administration of ethanolamine hydrochloride (24 mg of ethanolamine per kg of body weight) enhanced hepatocyte proliferation in regenerating rat livers after two-thirds hepatectomy without the administration of any growth factors. In the regenerating liver, hepatocyte proliferation may be initiated by an endogenous growth factor, but the supply of ethanolamine in circulation may not be sufficient for optimal hepatocyte proliferation; thus, the exogenous administration of ethanolamine may further enhance hepatocyte proliferation. Ethanolamine in circulation may be a humoral hepatotrophic factor.
Previously we reported that the proliferation of hepatocytes in primary culture was synergistically stimulated by a low molecular weight (LMW, <1,000) and a high molecular weight (HMW, >10,000) factor that were derived from the bovine small intestine mucosa, separated by their solubility in methanol (1). The LMW factor synergistically enhanced the basal level of stimulation by the HMW factor, while it had little effect when added alone. Since the LMW factor could enhance the level of DNA synthesis in hepatocytes that were stimulated by epidermal growth factor (EGF) or hepatocyte growth factor (HGF), it acts by amplifying the effects of a growth factor. Contrary to the in vitro result, the LMW factor could stimulate hepatocyte proliferation in regenerating livers even when added alone. We speculate that an endogenous growth factor(s) is probably produced in the animals with the regenerating liver, but the supply of the LMW factor is below the optimal level, thus the exogenous supply of the LMW factor can further stimulate hepatocyte proliferation. The LMW factor may function as a humoral hepatotrophic factor for liver regeneration.
We purified the LMW factor from the bovine small intestinal mucosa, and we identified it as ethanolamine (Etn). The commercially available Etn had effects identical to those of the LMW factor before purification; i.e., Etn synergistically stimulated the proliferation of hepatocytes in primary culture when added with the HMW factor, and it stimulated hepatocyte proliferation in the regenerating liver when administered alone. Etn can synergistically enhance DNA synthesis in hepatocytes when added with EGF, suggesting that it enhances the actions of hepatotrophic growth factors. Etn in circulation may function as a humoral hepatotrophic factor in the early stages of liver regeneration, if an endogenous growth factor which acts synergistically with Etn is supplied.
MATERIALS AND METHODS
Hepatocytes for primary cultures were prepared from 8- to 12-week-old male Sprague–Dawley rats by the perfusion method (2). The level of DNA synthesis in hepatocytes in primary culture was assayed by detecting the radioactivity of the incorporated [3H]thymidine (1). Hepatocytes were plated in a 24-well collagen-coated culture dish at 80,000 cells in 0.4 ml per well, and cultured in RPMI 1640 medium supplemented with 5% newborn calf serum (Mitsubishi Chemicals, Tokyo) at 37°C under 5% CO2 for 24 h. Then the medium was changed to one without the serum but with insulin (0.1 μM). The samples to be tested were added at this time. The medium and samples were renewed after 24 h of incubation. After another 24 h of incubation, the medium was changed to one without any supplements or samples. [3H]Thymidine (Amersham Japan, Tokyo) was added to the cells at a dose of 2 μCi per well (1 μCi = 37 kBq) and incubated for 2 h. The radioactivity in the hepatocytes was measured after washing the cells with 5% trichloroacetic acid/95% ethanol. The samples were assayed in duplicate.
Hepatocyte proliferation in the rats, after partial hepatectomy, was assayed by immunohistochemically detecting 5-bromodeoxyuridine (BrdUrd) incorporated into the nuclei of hepatocytes (1). The hydrochloride salt of Etn (Sigma) dissolved in saline (24 mg/4 ml per kg of body weight as free Etn) was intraperitoneally injected into rats within a minute after two-thirds hepatectomy. The injection of the same dose of Etn was repeated every 24 h. The BrdUrd solution (1) was intraperitoneally injected 22, 46, 70, or 166 h after hepatectomy. After a 2-h pulse, the liver was excised and processed for paraffin sectioning.
The bovine intestinal mucosa extract was prepared as described (1). The mucosa (500 g), collected from slaughtered animals and stored frozen at −80°C, was thawed in 2,000 ml of methanol and homogenized in a Waring blender. The homogenate was kept at 4°C for 6 h with occasional stirring and then filtered through a filter paper in a Buchner funnel. The residue on the filter paper was suspended in 1,000 ml of methanol and kept at 4°C for 12 h for another extraction. The filtrate of this suspension was combined with the previous one, and concentrated to 100 ml by heating to 70°C. To the concentrate, 100 ml of methanol was added, and the mixture was centrifuged to remove the insoluble materials. The supernatant was concentrated into a dense solution in a beaker in boiling water and was diluted to 50 ml with water. The precipitate that formed during storage at 4°C overnight was removed by centrifugation. The supernatant was kept at 4°C and used as the methanol-soluble fraction. One milliliter of such an extract yielded 120 mg of dried material. The residue retained in the funnel was suspended in 200 ml of phosphate-buffered saline and stirred at 0°C for 12 h. The suspension was centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was centrifuged at 100,000 × g for 60 min at 4°C. The supernatant was filtered through a 0.45-μm pore membrane. The filtrate was stored in aliquots at −20°C and used as the methanol-insoluble fraction. The extract used for the experiments in this report contained protein at 7.0 mg/ml. The methanol-soluble fraction was used as the LMW factor, while the methanol-insoluble fraction was used as the HMW factor. Etn (Wako, Tokyo) was used as a 1 M solution neutralized with HCl.
The components in the methanol-soluble fraction were fractionated by gel filtration chromatography through a Sephacryl S-100 column (5 × 30 cm, Pharmacia) equilibrated with pure water at a flow rate of 2 ml/min. The 10-ml fractions that contained the activity were pooled and concentrated in a rotary evaporator under reduced pressure at 40°C. The concentrate was subjected to isocratic separation through a C18 silica column (20 × 250 mm, Hibar, Merck-Cica, Tokyo) equilibrated with 2% methanol and 20 mM Tris⋅HCl (pH 7.4). The fractions with activity were pooled and concentrated under reduced pressure in a centrifugal evaporator at 45°C. The components in the concentrate were modified into phenylthiocarbamoyl derivatives by incubation at room temperature for 20 min with 10% phenylisothiocyanate/10% triethylamine/70% ethanol. The mixture was dried in a centrifugal vaporizer as above. The dried material was dissolved in 20% methanol. The phenylthiocarbamoyl derivatives were applied to a C18 silica column (4.6 × 250 mm) equilibrated with 4.5% CH3CN/0.1% CH3COOH/NH4OH (pH 5.4) at 40°C at a flow rate of 1 ml/min. The adsorbed substances were eluted in a gradient of CH3CN, which went up to 90%, over 40 min.
RESULTS
Purification of the LMW Factor.
As reported previously (1), the methanol-soluble fraction of the bovine small intestinal mucosa, which contains the LMW factor, could not stimulate DNA synthesis in hepatocytes in primary culture by itself. However, it synergistically enhanced the level of DNA synthesis when added together with the HMW factor (Fig. 1).
Figure 1.
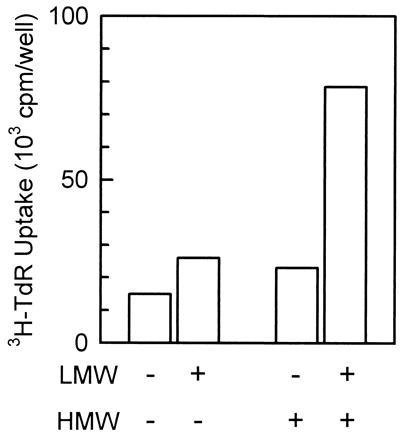
Synergistic stimulation of DNA synthesis of hepatocytes in primary culture by the LMW and HMW factors derived from the bovine small intestinal mucosa. The LMW factor (3%, vol/vol) and the HMW factor (1%, vol/vol) from the bovine small intestinal mucosa slightly stimulated DNA synthesis ([3H]thymidine uptake) when each of them was independently added to rat hepatocytes in primary culture. However, a synergistic increase in stimulation was seen when they were added simultaneously. The LMW factor and the HMW factor are referred to as LMW and HMW, respectively. All bars are the mean of duplicate assays.
The components in the methanol-soluble fraction were separated first by gel chromatography through a Sephacryl S-100 column (Fig. 2). The fractions that had the LMW factor activity were pooled and subjected to reversed-phase chromatography. The activity eluted in the unadsorbed fractions, indicating the existence of a charged group in the molecule. Since the unadsorbed fractions reacted with ninhydrin, the molecules in the fractions were mixed with phenylisothiocyanate under alkaline conditions, modifying them into phenylthiocarbamoyl derivatives. After the reaction, the LMW factor activity was not detected, indicating that the molecule responsible for LMW factor activity possesses an amino group, and its modification with phenylisothiocyanate results in the loss of its activity (data not shown). The modified molecules in each fraction were separated by reversed-phase chromatography on a C18 silica column, so a peak that specifically appears in the fractions that have LMW factor activity could be identified. One distinctive peak was found in these fractions.
Figure 2.
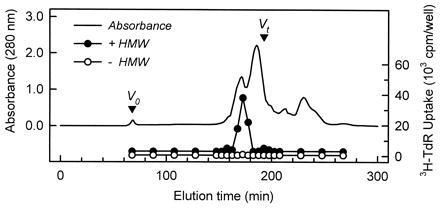
Profile of gel filtration chromatography of the LMW factor. The LMW factor (10 ml) was loaded into a Sephacryl S-100 column (5 × 30 cm) equilibrated with pure water at a flow rate of 2 ml/min. Fractions (10 ml) were assayed for their activity that enhances the DNA synthesis in hepatocytes. The fractions (3%, vol/vol) were added to the culture with or without the HMW factor (1%, vol/vol). DNA synthesis was monitored by the radioactivity of [3H]thymidine incorporated into the cells. The HMW factor is referred to as HMW.
Structure of the LMW Factor.
The structure of the substance in the peak was analyzed by NMR and liquid chromatography–mass spectrometry (LC-MS). The NMR analysis showed that the substance constituting the peak had two methylene groups, as indicated by the chemical shifts at 3.65 and 3.69 ppm. The LC-MS analysis showed that M + 1 was 197, indicating the molecular mass of the substance before modification is 61 Da. These findings indicated that the substance in the peak was most likely to be Etn. This idea was also supported by the fact that the substance in the collected peak had an elution time identical to that of phenylthiocarbamoyl ethanolamine, as shown by the analysis of phenylthiocarbamoyl amino acids (Fig. 3).
Figure 3.
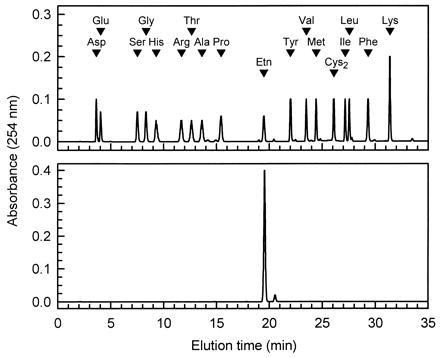
Analysis of the purified phenylthiocarbamoyl derivative of the LMW factor by the phenylthiocarbamoyl-amino acid method (3). When compared with phenylthiocarbamoyl amino acid standards (1 nmol of each, Upper), the purified substance (Lower) eluted at the position of phenylthiocarbamoyl-Etn.
Stimulation of Proliferation of Hepatocytes in Primary Culture by Etn.
Commercially available Etn was used to identify Etn as the LMW factor. Etn enhanced DNA synthesis in hepatocytes in primary culture when added with the HMW factor, with the ED50 being 20 μM. When Etn was added by itself, its effect was much less than in the presence of the HMW factor (Fig. 4 Left). Etn could synergistically enhance the level of stimulation induced by EGF in the same range of concentrations (Fig. 4 Right). These results indicate that the mode of action of Etn is identical to that of the LMW factor in the methanol-soluble fraction.
Figure 4.
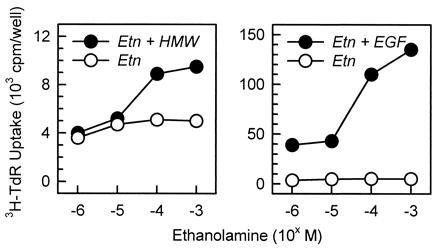
Synergistic stimulation of DNA synthesis of hepatocytes in primary culture by a combination of Etn and either the HMW factor or EGF. The HMW factor and EGF were added to the culture at 1% vol/vol (Left) and 40 ng/ml (Right), respectively, and Etn was 40 μM. DNA synthesis was monitored by the radioactivity of [3H]thymidine incorporated into the cells. The HMW factor is referred to as HMW. All points are the mean of duplicate assays.
Stimulation of Proliferation in Hepatocytes in the Regenerating Liver by Etn.
Because the methanol-soluble fraction of the bovine intestinal mucosa enhanced hepatocyte proliferation in regenerating rat livers (1), Etn was assayed for its activity in the in vivo system. The hydrochloride salt of Etn dissolved in saline (24 mg/4 ml per kg of body weight as free Etn) was intraperitoneally injected into rats after two-thirds hepatectomy, as described in Materials and Methods. The percentage of BrdUrd-labeled nuclei in the hepatocytes was significantly higher in the Etn-injected rats than in the saline-injected rats (Fig. 5). The stimulation of DNA synthesis by Etn was not seen after 48 h after hepatectomy, even though Etn was injected every 24 h. These results indicate that Etn enhances the proliferation of hepatocytes in the regenerating liver, but it does not disturb the cycle of hepatocyte proliferation in liver regeneration.
Figure 5.
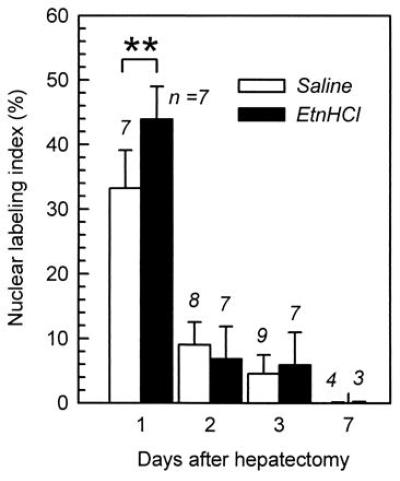
Enhancement of the rate of hepatocyte proliferation in the regenerating liver by the intraperitoneal administration of Etn. The rate of hepatocyte proliferation was monitored by immunohistochemically detecting BrdUrd incorporation into the nuclei of hepatocytes. The hydrochloride salt of Etn (24 mg/kg of body weight as free Etn) was intraperitoneally injected every 24 h into rats after two-thirds hepatectomy. Saline was intraperitoneally injected into the control group. The numbers of rats in each group are indicated at the top of the bars. ∗∗, P < 0.01 (t test).
DISCUSSION
The aim of our investigation was to identify the nature of the LMW hepatotrophic factor that was found in a bovine small intestinal mucosa extract. As described in this report, it was purified and identified as Etn. Furthermore, Etn acts as a hepatotrophic factor not only in assays in vitro in primary hepatocyte cultures but also in assays in vivo of liver regeneration in two-thirds hepatectomized rats. The Etn responsiveness of cultured cells was first described by Kano-Sueoka in neoplastic mammary cells (4). Some other types of cells such as keratinocytes (5) and hybridomas (6) have since been reported as Etn-responsive cells. Although it was known that exogenously added Etn prolongs rat hepatocyte cultures (7), details of the responses of hepatocytes to Etn were not known. We discuss the mechanism of the stimulation of hepatocyte proliferation by Etn and the relevance of exogenously administered Etn to liver regeneration in vivo.
Although how exogenously added Etn stimulates proliferation of hepatocytes is not clear, the fact that an exogenous load of Etn increases the amount of phosphatidylethanolamine (PtdEtn) in the plasma membrane of Etn-responsive cells (8) suggests that the effect of Etn could be attributed to the stimulation of phospholipid synthesis. In hepatocytes, most PtdEtn can be synthesized by combining CDP-Etn with 1,2-diacylglycerol (9, 10). In this pathway, both the availability of Etn and 1,2-diacylglycerol and the activity of enzymes, choline (ethanolamine) kinase and CTP:phosphoethanolamine cytidylyltransferase, are required (11). Among these, the supply of Etn seems to primarily influence PtdEtn synthesis in hepatocytes (12). PtdEtn can also be synthesized by the decarboxylation of phosphatidylserine (13) and by a base-exchange reaction (14). These pathways, however, are not the major routes of PtdEtn synthesis in hepatocytes (9, 12, 14).
The stimulation of PtdEtn synthesis in hepatocytes may change the amount of phosphatidylcholine (PtdCho), since a substantial amount of PtdCho in hepatocytes is synthesized by the addition of methyl groups to PtdEtn by the action of PtdEtn methyltransferase using S-adenosylmethionine as a methyl donor, with the rate of PtdCho synthesis via this pathway being 20–40% of that of the CDP-choline/1,2-diacylglycerol pathway (11). PtdEtn and PtdCho synthesized in this way might be required for modulation of hepatocyte proliferation. As seen in other cell systems, they might activate protein kinase C systems that are known to be important for activation of cellular phenomena, because either the change in the PtdEtn/PtdCho ratio in the plasma membrane induces the translocation of protein kinase C from the cytoplasm to the plasma membrane, resulting in its activation (15), or an increase of PtdCho in the plasma membrane provides a substrate for phospholipases to produce signal mediators, such as phosphatidic acid and diacylglycerol (16, 17). Meanwhile, by the action of phospholipase D, choline is also liberated from PtdCho. In growth factor-stimulated NIH 3T3 cells, the choline liberated is phosphorylated by choline kinase to produce phosphocholine (18). This step is thought to be essential for the activation of Raf-1 and MAP kinases, which are thought to be involved in the transmission of signals to the nucleus (18, 19). Consequently, synergistic stimulation with Etn of the hepatocyte proliferation might be due to the enhanced production of these mediators in the hepatocytes that have been stimulated by a growth factor or factors.
Similar regulatory patterns of hepatocyte proliferation by a LMW and a HMW substance are seen when norepinephrine (20), estradiol (21), or triiodothyronine (22) was added to hepatocytes with EGF. These three substances do not significantly stimulate hepatocyte proliferation by themselves, but they cause the synergistic stimulation of proliferation when added with EGF. Stimulation by these substances is mediated by their specific receptors (23). Thus, synergistic stimulation by a combination of these molecules and EGF seems to be due to the stimulation of two distinct signal transducing pathways. The HMW factor in this report is likely to be a molecule similar to growth factors, based on the facts that its molecular mass is 30 kDa, it is heat labile, and it can be replaced by EGF. Therefore, as far as the HMW factor is concerned, the action of Etn is very similar to that of the above three molecules. However, the fact that Etn acts as an activator of any cellular phenomena by transmitting its signal into cells through a specific receptor has not been described previously. Accordingly, the mechanism of the action of Etn is probably distinct from the mechanisms of the above three. It is more likely that Etn exerts its effect by enhancing the synthesis of phospholipids.
The rate of hepatocyte proliferation in the regenerating liver was enhanced by the intraperitoneal administration of Etn. The results in primary hepatocyte cultures showed that the HMW factor is required before the effect of Etn on the stimulation of hepatocyte proliferation in the regenerating liver is seen. This might be explained by assuming that the HMW factor, or a growth factor(s) such as heparin-binding EGF (24), HGF (25), transforming growth factor α (26), or acidic fibroblast growth factor (27), is produced in the regenerating liver so that hepatocyte proliferation is adequately initiated on time. On the other hand, Etn is provided from circulation, but its concentration is below the level required to exert maximal stimulation: 100 μM Etn is required as shown in the in vitro assay. The concentration of free Etn in circulation in rats is maintained at 20 μM in the serum, and transiently increases to 30 μM 4 h after hepatectomy (our unpublished data), and to 50 μM 22 h after hepatectomy (12). Therefore, exogenous Etn further stimulates hepatocyte proliferation in the regenerating liver.
Later than 48 h after hepatectomy, Etn did not enhance the incorporation of BrdUrd into hepatocyte nuclei. Since the hepatocytes in the liver after two-thirds hepatectomy show a major peak of proliferation at 24 h (28, 29), the majority of hepatocytes are no longer in the proliferating stage after more than 48 h. Thus, the above data indicate that Etn does not affect the proliferation of hepatocytes that have passed the progression period of proliferation. The proliferation of hepatocytes is thought to be controlled by growth factors (see refs. 23 and 30). Etn in circulation may function as a humoral hepatotrophic factor that modulates the rate of hepatocyte proliferation that has been stimulated by a growth factor or factors.
Recently, it was reported that a hepatotrophic factor in livers of weanling pigs that enhances the actions of HGF and transforming growth factor α (TGF-α) in vitro was identified as glycerophosphoethanolamine (31). It was also reported that Etn, a moiety of glycerophosphoethanolamine, enhances the level of DNA synthesis in hepatocytes in primary culture that were stimulated by the growth factors HGF and TGF-α. The conclusion of these workers is identical to that of our independent investigation, although the starting materials for purification, the purified substances, and the growth factor we used were different. In spite of these identical conclusions, our interpretation of the results as to the relevance of Etn to liver regeneration in vivo is different. Nelson et al. (31) speculate that administration of Etn will not modify the rate of liver regeneration in vivo unless Etn levels in circulation drop due to nutritional or metabolic abnormalities. They base their perception on the fact that the lowest Etn concentration required for a maximal response of hepatocyte proliferation in vitro (30 μM in their assays) is lower than the levels of free Etn in circulation during liver regeneration (range as described above). Our data, on the other hand, indicate that Etn can significantly enhance the proliferation of hepatocytes in regenerating livers of rats under our experimental conditions. We speculate that the concentrations of free Etn in circulation after hepatectomy are not yet optimal and cannot attain the maximal stimulation of hepatocyte proliferation. Our estimation of an Etn concentration at which the hepatocyte response in vitro reaches a plateau (100 μM) supports this idea. The difference in optimal levels of Etn in our in vitro assays might derive from a different pulsing time for [3H]thymidine uptake, overnight in their system vs. 2 h in ours. Longer pulsing periods usually result in higher levels of [3H]thymidine uptake at lower concentrations of effectors.
It should also be considered that estimations of optimal Etn levels made by in vitro assays in which all hepatocytes are equally exposed to the medium might not accurately reflect the in vivo situation. Hepatocytes in liver might not be exposed to an equal concentration of Etn due to the liver’s structure. There might be a gradient of available Etn concentrations along the route of blood flow in the hepatic acinus, which starts at the portal venous system and ends at the hepatic venous system. Hepatocytes located closer to the inlets of circulation (portal venous area) would take up Etn and lower circulating Etn concentrations. Therefore, hepatocytes located at a more remote location from the inlets (hepatic venous area) would be exposed to lower Etn concentrations. Such microenvironments in livers might cause the limited availability of circulating Etn, resulting in a demonstration of effectiveness of an exogenous administration of Etn. Our results, which show the effectiveness of Etn on hepatocyte proliferation in regenerating livers, do not reflect the possibility of accumulation of proliferating hepatocytes at local areas, because the BrdUrd labeling indices were obtained on the basis of photographs taken randomly to cover as wide an area as possible.
In conclusion, exogenous Etn can modulate the rate of hepatocyte proliferation in regenerating livers. However more detailed investigations are required for an understanding of the mechanism of the in vivo influence of Etn on hepatocyte proliferation.
Acknowledgments
We are grateful to Dr. Katsumi Ajisaka and Dr. Ichiro Matsuo for their cooperation for the NMR analysis. We are also grateful to Dr. Tamiko Kano-Sueoka for critical reading of the manuscript and discussion.
ABBREVIATIONS
- LMW
low molecular weight
- HMW
high molecular weight
- EGF
epidermal growth factor
- HGF
hepatocyte growth factor
- Etn
ethanolamine
- PtdEtn
phosphatidylethanolamine
- PtdCho
phosphatidylcholine
- BrdUrd
5-bromodeoxyuridine
References
- 1.Sasaki, H., Nemoto, A., Kume, H., Narisawa, S. & Takahashi, N. (1997) In Vitro Cell. Dev. Biol. Anim., in press. [DOI] [PubMed]
- 2.Takahashi N, Kumanaka H, Takagi H, Mori N. In Vitro Cell Dev Biol. 1989;25:365–372. doi: 10.1007/BF02624600. [DOI] [PubMed] [Google Scholar]
- 3.Heinrikson R L, Meredith S S. Anal Biochem. 1984;136:65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- 4.Kano-Sueoka T, Cohen D M, Yamaizumi Z, Nishimura S, Mori M, Fujiki H. Proc Natl Acad Sci USA. 1979;76:5741–5744. doi: 10.1073/pnas.76.11.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao M C, Walthal B J, Ham R G. J Cell Physiol. 1982;110:219–229. doi: 10.1002/jcp.1041100217. [DOI] [PubMed] [Google Scholar]
- 6.Murakami H, Masui H, Sato G H, Sueoka N, Chow T P, Kano-Sueoka T. Proc Natl Acad Sci USA. 1982;79:1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki M, Bai L, Namba M. Res Exp Med. 1991;191:77–83. doi: 10.1007/BF02576661. [DOI] [PubMed] [Google Scholar]
- 8.Kano-Sueoka T, Errick J E. Exp Cell Res. 1981;136:137–145. doi: 10.1016/0014-4827(81)90045-8. [DOI] [PubMed] [Google Scholar]
- 9.Tijburg L B M, Geelen M J H, VanGolde L M G. Biochem Biophys Res Commun. 1989;160:1275–1280. doi: 10.1016/s0006-291x(89)80141-x. [DOI] [PubMed] [Google Scholar]
- 10.Arthur G, Page L. Biochem J. 1991;273:121–125. doi: 10.1042/bj2730121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundler R, Akesson B. J Biol Chem. 1975;250:3359–3367. [PubMed] [Google Scholar]
- 12.Houweling M, Tijburg L B M, Vaartjes J, VanGolde L M G. Biochem J. 1992;283:55–61. doi: 10.1042/bj2830055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voelker D R. Proc Natl Acad Sci USA. 1984;81:2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundler R, Akesson B, Nilsson A. FEBS Lett. 1974;43:303–307. doi: 10.1016/0014-5793(74)80667-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishizuka Y. Nature (London) 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 16.Exton J H. J Biol Chem. 1990;265:1–4. [PubMed] [Google Scholar]
- 17.Nishizuka Y. Nature (London) 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez B, DelPeso L, Montaner S, Esteve P, Lacal J C. J Cell Biochem. 1995;57:141–149. doi: 10.1002/jcb.240570114. [DOI] [PubMed] [Google Scholar]
- 19.Tomono M, Crilly K S, Kiss Z. Biochem Biophys Res Commun. 1995;213:980–985. doi: 10.1006/bbrc.1995.2225. [DOI] [PubMed] [Google Scholar]
- 20.Cruise J L, Cotecchia S, Michalopoulos G. J Cell Physiol. 1986;127:39–44. doi: 10.1002/jcp.1041270106. [DOI] [PubMed] [Google Scholar]
- 21.Ni N, Yager J D. Hepatology. 1994;19:183–192. [PubMed] [Google Scholar]
- 22.Francavilla A, Carr B I, Azzarone A, Polimeno L, Wang Z, VanThiel D H, Subbotin V, Prelich J G, Starzl T E. Hepatology. 1994;20:1237–1241. [PubMed] [Google Scholar]
- 23.Michalopoulos G K. FASEB J. 1990;4:176–187. [PubMed] [Google Scholar]
- 24.Kiso S, Kawata S, Tamura S, Higashiyama S, Ito N, Tsushima H, Taniguchi N, Matsuzawa Y. Hepatology. 1995;22:1584–1590. [PubMed] [Google Scholar]
- 25.Kinoshita T, Hirao S, Matsumoto K, Nakamura T. Biochem Biophys Res Commun. 1991;177:330–335. doi: 10.1016/0006-291x(91)91987-n. [DOI] [PubMed] [Google Scholar]
- 26.Mead J E, Fausto N. Proc Natl Acad Sci USA. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan M, Huan J, Mannson P, Yasumitsu H, Carr B, McKeehan W. Proc Natl Acad Sci USA. 1989;86:7432–7436. doi: 10.1073/pnas.86.19.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grisham J W. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- 29.Rabes H W, Wirshing R, Tuczk H V, Iseler G. Cell Tissue Kinet. 1976;6:517–532. doi: 10.1111/j.1365-2184.1976.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 30.Fausto N. Prog Growth Factor Res. 1991;3:218–234. doi: 10.1016/0955-2235(91)90008-r. [DOI] [PubMed] [Google Scholar]
- 31.Nelson C, Moffat B, Jacobson N, Henzel W J, Stults J T, King K L, McMurtrey A, Vandelen R, Spencer A A. Exp Cell Res. 1996;229:20–26. doi: 10.1006/excr.1996.0339. [DOI] [PubMed] [Google Scholar]


