Abstract
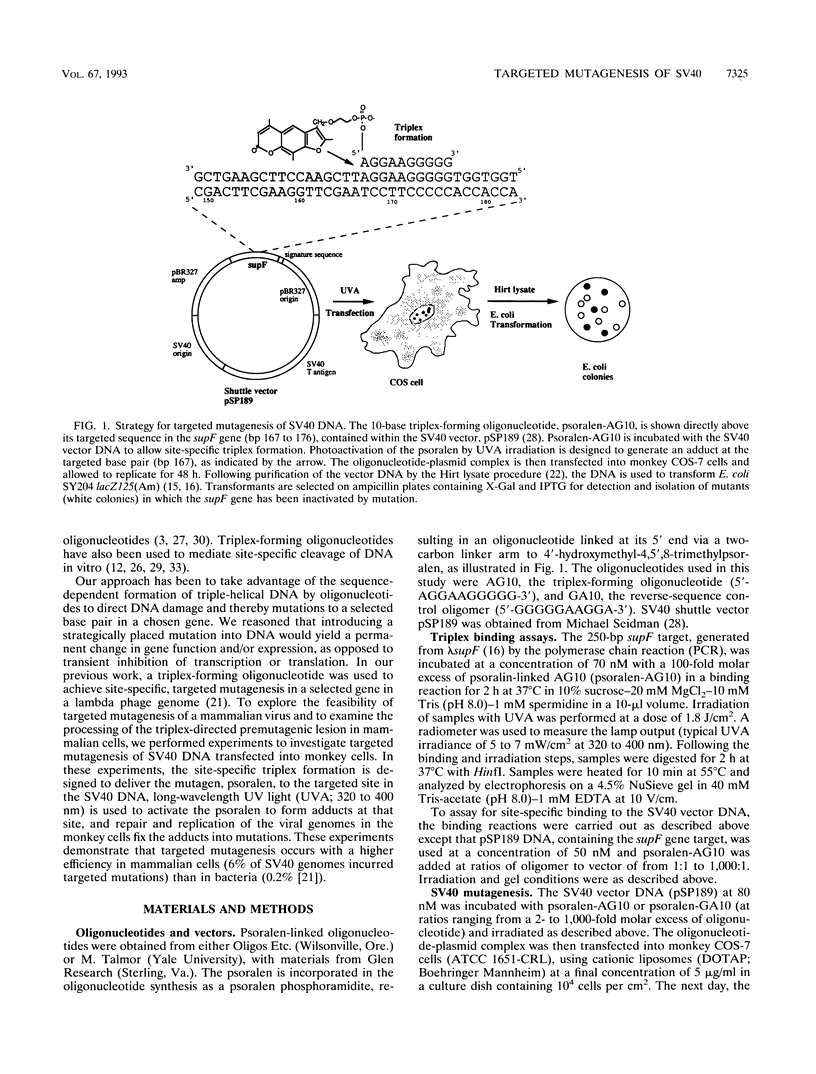
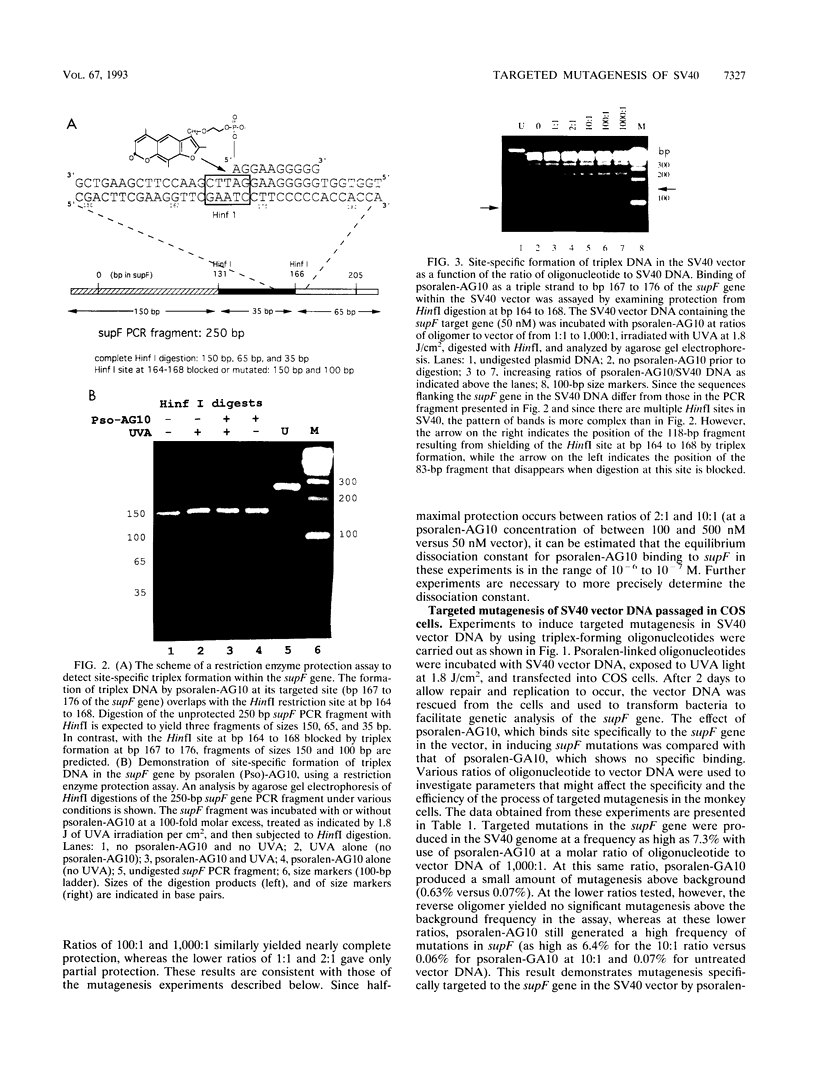
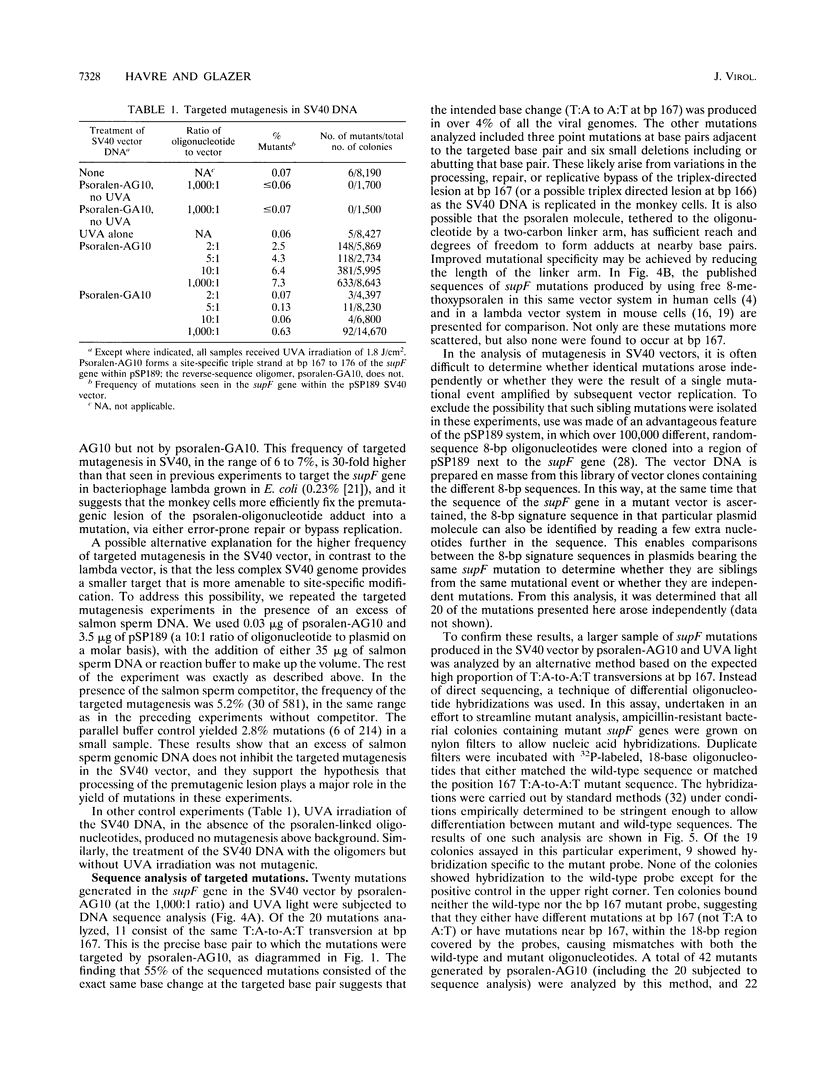
Triple-helical DNA can be formed by oligonucleotides that bind as third strands of DNA in a sequence-specific manner in the major groove in homopurine/homopyrimidine stretches in duplex DNA. Such triple helix-forming oligonucleotides have been used to inhibit gene expression by blocking transcription factor access to promoter sites in transient expression assays. In an alternative approach to genetic manipulation using triplex DNA, we show that triplex-forming oligonucleotides can be used to produce site-specific, targeted mutations in a viral genome in order to achieve a permanent, heritable effect on gene function and expression. We use a triplex-forming oligonucleotide linked to a psoralen derivative at its 5' end to achieve targeted mutagenesis in a simian virus 40 (SV40) vector genome. Site-specific triplex formation delivers the psoralen to the targeted site in the SV40 DNA. Photoactivation of the psoralen yields adducts and thereby mutations at that site. Mutations were produced in the target gene in over 6% of the viral genomes. DNA sequence analysis of the mutations in the target gene showed that all were in the targeted region, and 55% were found to be the same T:A-to-A:T transversion precisely at the targeted base pair. In control experiments, no mutagenesis above the background frequency in the assay was produced by a non-triplex-forming, psoralen-linked oligonucleotide unless a vast excess of this oligonucleotide was used, demonstrating the specificity of the targeted mutagenesis. This frequency of targeted mutagenesis of SV40 in monkey cells represents a 30-fold increase relative to similar experiments using lambda phage in bacteria, suggesting that fixation of the triplex-directed lesion into a mutation occurs more efficiently in mammalian cells. If the ability to reproducibly and predictably target mutations to sites in viral DNA in vitro by using modified oligonucleotides can be extended to DNA in vivo, this approach may prove useful as a technique for gene therapy, as a strategy for antiviral therapeutics, and as a tool for genetic engineering.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Beal P. A., Dervan P. B. The influence of single base triplet changes on the stability of a pur.pur.pyr triple helix determined by affinity cleaving. Nucleic Acids Res. 1992 Jun 11;20(11):2773–2776. doi: 10.1093/nar/20.11.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume S. W., Gee J. E., Shrestha K., Miller D. M. Triple helix formation by purine-rich oligonucleotides targeted to the human dihydrofolate reductase promoter. Nucleic Acids Res. 1992 Apr 11;20(7):1777–1784. doi: 10.1093/nar/20.7.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Nachmansson N. Psoralen adducts in a shuttle vector plasmid propagated in primate cells: high mutagenicity of DNA cross-links. Carcinogenesis. 1987 Dec;8(12):1923–1927. doi: 10.1093/carcin/8.12.1923. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Barbier C., Chassignol M., Thuong N. T., Hélène C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Thuong N. T., Hélène C. Inhibition of restriction endonuclease cleavage via triple helix formation by homopyrimidine oligonucleotides. Biochemistry. 1989 Dec 12;28(25):9617–9619. doi: 10.1021/bi00451a011. [DOI] [PubMed] [Google Scholar]
- Giovannangéli C., Rougée M., Garestier T., Thuong N. T., Hélène C. Triple-helix formation by oligonucleotides containing the three bases thymine, cytosine, and guanine. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8631–8635. doi: 10.1073/pnas.89.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Sarkar S. N., Chisholm G. E., Summers W. C. DNA mismatch repair detected in human cell extracts. Mol Cell Biol. 1987 Jan;7(1):218–224. doi: 10.1128/mcb.7.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Sarkar S. N., Summers W. C. Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1041–1044. doi: 10.1073/pnas.83.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev M., Praseuth D., Guieysse A. L., Robin P., Thuong N. T., Hélène C., Harel-Bellan A. Inhibition of gene expression by triple helix-directed DNA cross-linking at specific sites. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3501–3505. doi: 10.1073/pnas.90.8.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev M., Praseuth D., Robin P., Hemar A., Saison-Behmoaras T., Dautry-Varsat A., Thuong N. T., Hélène C., Harel-Bellan A. A triple helix-forming oligonucleotide-intercalator conjugate acts as a transcriptional repressor via inhibition of NF kappa B binding to interleukin-2 receptor alpha-regulatory sequence. J Biol Chem. 1992 Feb 15;267(5):3389–3395. [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990 Jan 11;18(1):157–161. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havre P. A., Gunther E. J., Gasparro F. P., Glazer P. M. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7879–7883. doi: 10.1073/pnas.90.16.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Seidman M. M. Use of supF, an Escherichia coli tyrosine suppressor tRNA gene, as a mutagenic target in shuttle-vector plasmids. Mutat Res. 1989 Mar-May;220(2-3):61–72. doi: 10.1016/0165-1110(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Bhan P., Cushman C. D., Trapane T. L. Recognition of a guanine-cytosine base pair by 8-oxoadenine. Biochemistry. 1992 Jul 28;31(29):6788–6793. doi: 10.1021/bi00144a020. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris C. N., Seidman M. M. A signature element distinguishes sibling and independent mutations in a shuttle vector plasmid. Gene. 1992 Aug 1;117(1):1–5. doi: 10.1016/0378-1119(92)90482-5. [DOI] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S. A., Doucette-Stamm L. A., Riba L., Housman D. E., Dervan P. B. Site-specific cleavage of human chromosome 4 mediated by triple-helix formation. Science. 1991 Dec 13;254(5038):1639–1642. doi: 10.1126/science.1836279. [DOI] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. L., Krawczyk S. H., Matteucci M. D., Toole J. J. Triple helix formation inhibits transcription elongation in vitro. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10023–10026. doi: 10.1073/pnas.88.22.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]