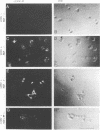
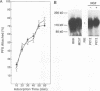
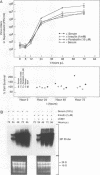
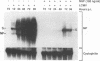
Abstract
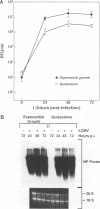
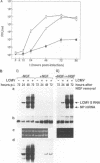
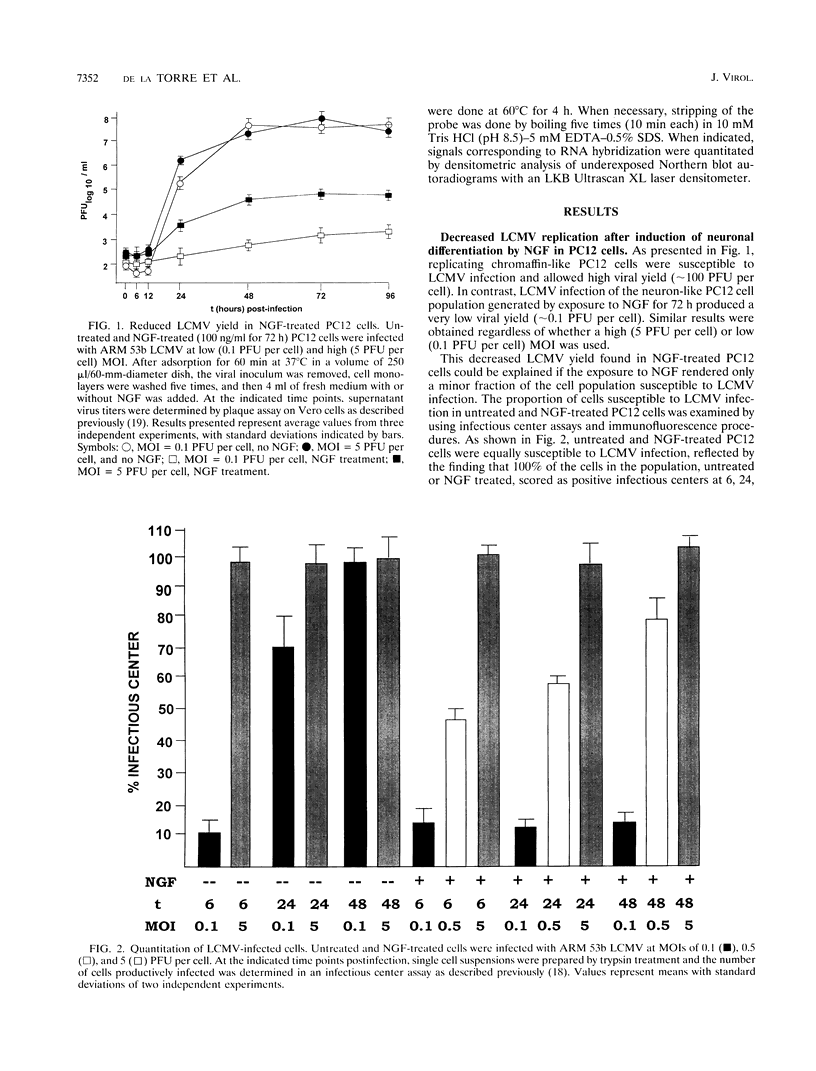
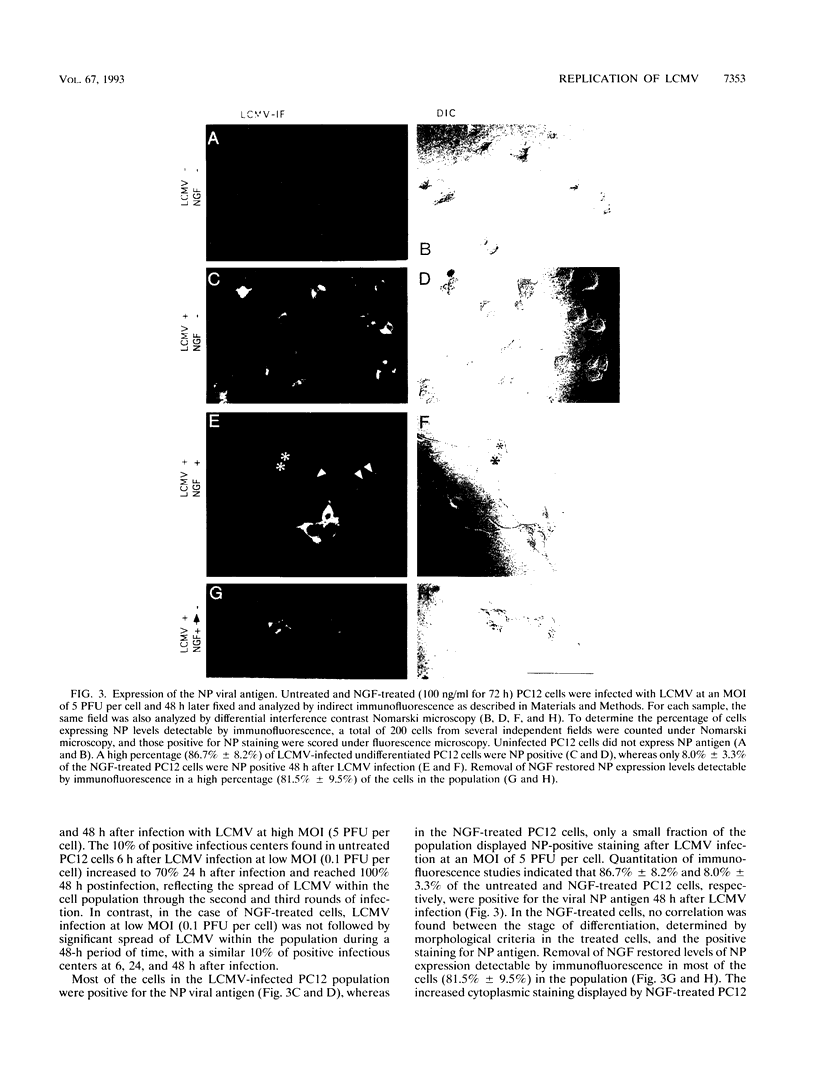
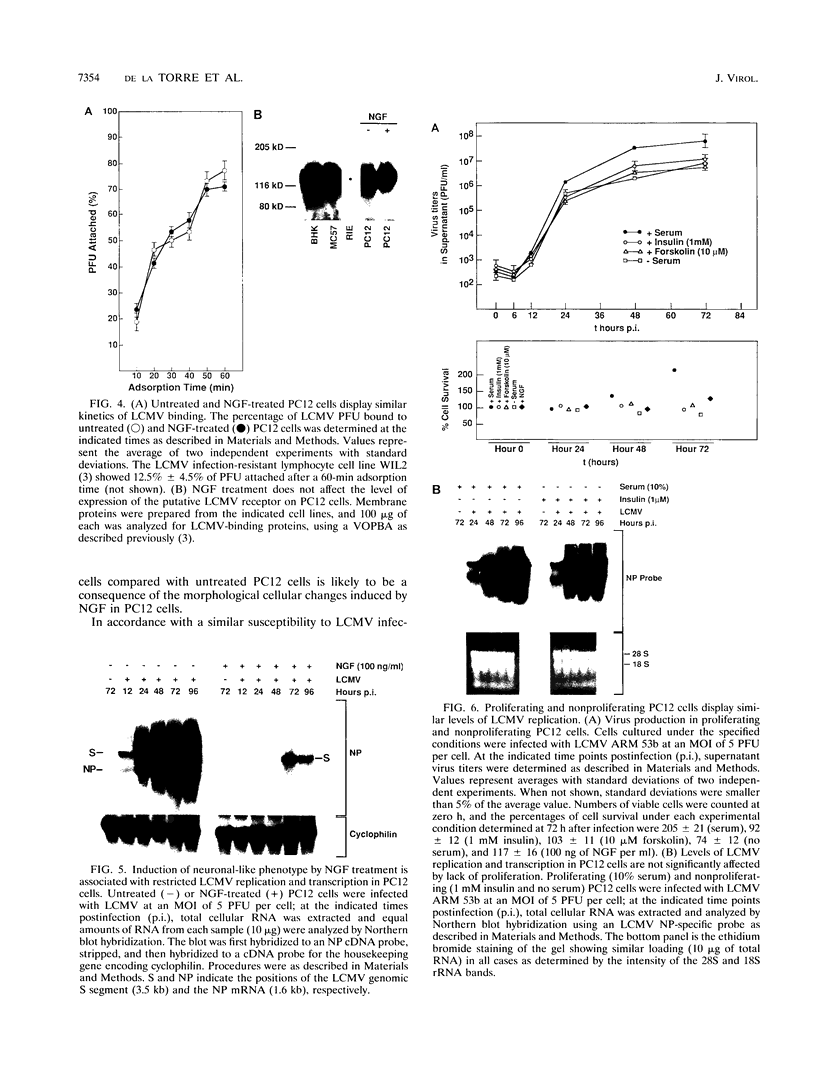
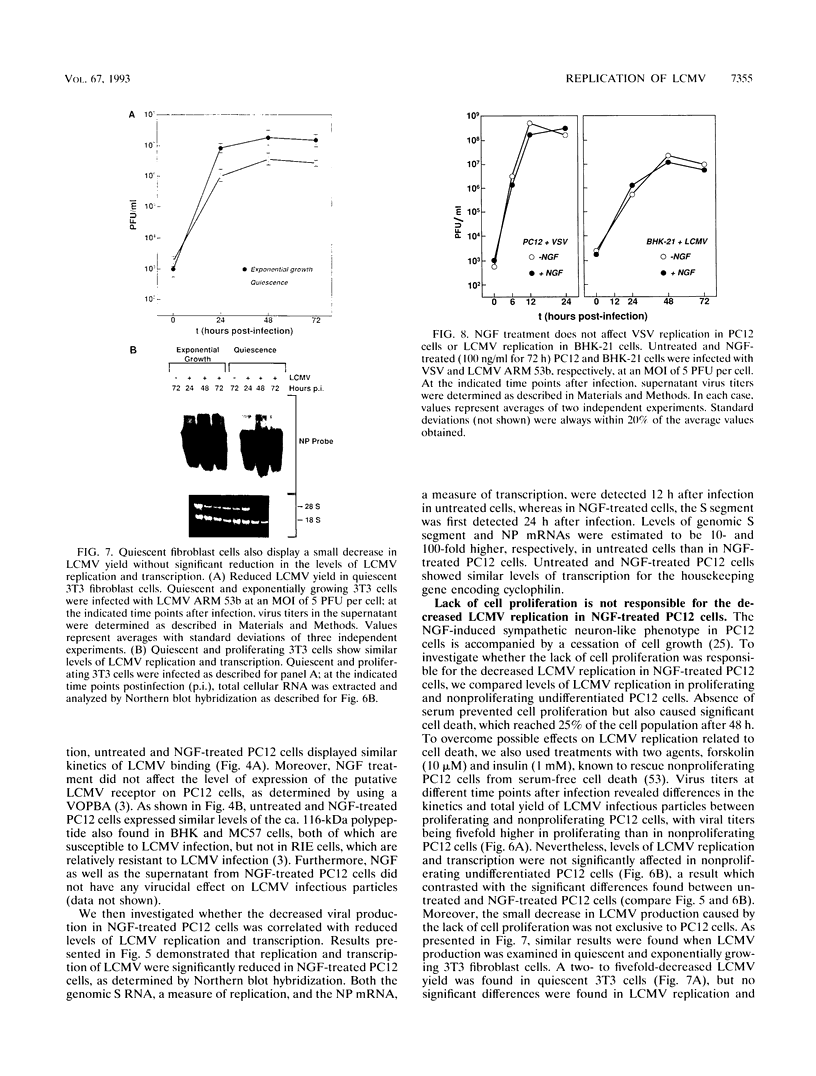
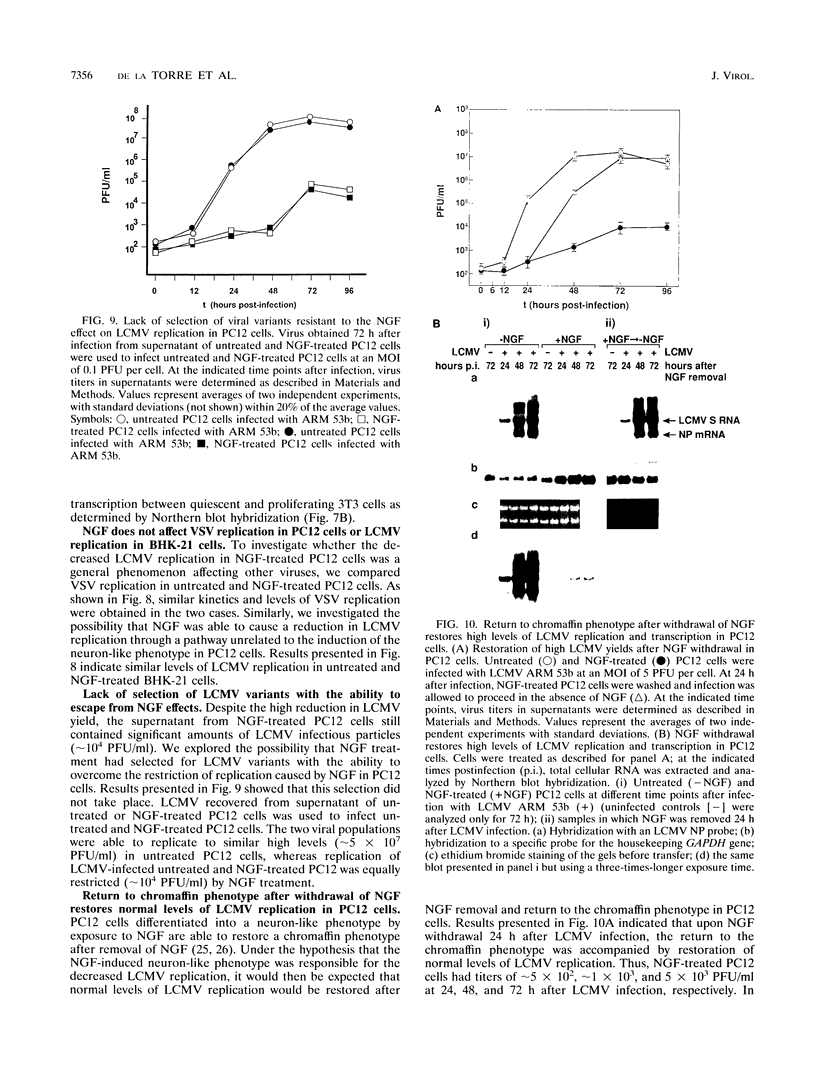
We have investigated the replication of lymphocytic choriomeningitis virus (LCMV) before and after the nerve growth factor (NGF)-induced transdifferentiation of PC12 cells from the chromaffin to the neuron-like phenotype. Untreated and NGF-treated cells were equally susceptible to LCMV infection; however, the viral yield was found to be 1,000-fold lower in NGF-differentiated PC12 cells. The reduced viral yield correlated with restricted LCMV replication and transcription within the infected cell, which was not caused by the lack of cell proliferation in the NGF-treated cells but rather was related to the induction or changes in expression levels of specific gene product(s) associated with the cell commitment to a neuronal phenotype. The return to the chromaffin phenotype after withdrawal of NGF restored normal LCMV yields as well as levels of viral replication and transcription. The finding of reduced viral replication in terminally differentiated neuronal cells has important implications for understanding the mechanism by which neurotropic viruses, such as LCMV, are able to establish a long-term persistent infection in the central nervous system in the absence of severe pathological changes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Axel R. Molecular probes for the development and plasticity of neural crest derivatives. Cell. 1985 Sep;42(2):649–662. doi: 10.1016/0092-8674(85)90122-9. [DOI] [PubMed] [Google Scholar]
- Borrow P., Oldstone M. B. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992 Dec;66(12):7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Burstein D. E., Greene L. A. Evidence for RNA synthesis-dependent and -independent pathways in stimulation of neurite outgrowth by nerve growth factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6059–6063. doi: 10.1073/pnas.75.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane C., McLain L., Dimmock N. J. Intracellular stability of the interfering activity of a defective interfering influenza virus in the absence of virus multiplication. Virology. 1987 Aug;159(2):259–264. doi: 10.1016/0042-6822(87)90463-6. [DOI] [PubMed] [Google Scholar]
- Chebath J., Benech P., Revel M., Vigneron M. Constitutive expression of (2'-5') oligo A synthetase confers resistance to picornavirus infection. Nature. 1987 Dec 10;330(6148):587–588. doi: 10.1038/330587a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem R. J., Fechheimer M., Miller L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991 Nov 29;254(5036):1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Kennedy P. G. Modulation of herpes simplex virus (HSV) infection of cultured neuronal cells by nerve growth factor and antibody to HSV. Brain. 1989 Oct;112(Pt 5):1277–1294. doi: 10.1093/brain/112.5.1277. [DOI] [PubMed] [Google Scholar]
- DePolo N. J., Giachetti C., Holland J. J. Continuing coevolution of virus and defective interfering particles and of viral genome sequences during undiluted passages: virus mutants exhibiting nearly complete resistance to formerly dominant defective interfering particles. J Virol. 1987 Feb;61(2):454–464. doi: 10.1128/jvi.61.2.454-464.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Cytomegalovirus causes a latent infection in undifferentiated cells and is activated by induction of cell differentiation. J Exp Med. 1981 Nov 1;154(5):1636–1651. doi: 10.1084/jem.154.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Ehrnst A. Growth phase related loss of measles virus surface-associated antigens and cytotoxic susceptibility in persistently infected cells. J Gen Virol. 1979 Dec;45(3):547–556. doi: 10.1099/0022-1317-45-3-547. [DOI] [PubMed] [Google Scholar]
- Fazakerley J. K., Southern P., Bloom F., Buchmeier M. J. High resolution in situ hybridization to determine the cellular distribution of lymphocytic choriomeningitis virus RNA in the tissues of persistently infected mice: relevance to arenavirus disease and mechanisms of viral persistence. J Gen Virol. 1991 Jul;72(Pt 7):1611–1625. doi: 10.1099/0022-1317-72-7-1611. [DOI] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hotchin J., Seegal R. Virus-induced behavioral alteration of mice. Science. 1977 May 6;196(4290):671–674. doi: 10.1126/science.854742. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Krause D., Friedman R. M., Silverman R. H. Induction of ppp(A2'p)nA-dependent RNase in murine JLS-V9R cells during growth inhibition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4954–4958. doi: 10.1073/pnas.80.16.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Geckeler R., Oldstone M. B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989 Dec;70(Pt 12):3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- Krause D., Silverman R. H., Jacobsen H., Leisy S. A., Dieffenbach C. W., Friedman R. M. Regulation of ppp(A2'p)nA-dependent RNase levels during interferon treatment and cell differentiation. Eur J Biochem. 1985 Feb 1;146(3):611–618. doi: 10.1111/j.1432-1033.1985.tb08695.x. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Norrby E. Persistence of RNA viruses in the central nervous system. Annu Rev Microbiol. 1986;40:159–184. doi: 10.1146/annurev.mi.40.100186.001111. [DOI] [PubMed] [Google Scholar]
- Kumar R., Choubey D., Lengyel P., Sen G. C. Studies on the role of the 2'-5'-oligoadenylate synthetase-RNase L pathway in beta interferon-mediated inhibition of encephalomyocarditis virus replication. J Virol. 1988 Sep;62(9):3175–3181. doi: 10.1128/jvi.62.9.3175-3181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kündig T. M., Hengartner H., Zinkernagel R. M. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol. 1993 Mar 15;150(6):2316–2321. [PubMed] [Google Scholar]
- Lehmann-Grube F. Portraits of viruses: arenaviruses. Intervirology. 1984;22(3):121–145. doi: 10.1159/000149543. [DOI] [PubMed] [Google Scholar]
- Levi A., Biocca S., Cattaneo A., Calissano P. The mode of action of nerve growth factor in PC12 cells. Mol Neurobiol. 1988 Fall;2(3):201–226. doi: 10.1007/BF02935346. [DOI] [PubMed] [Google Scholar]
- Levine B., Huang Q., Isaacs J. T., Reed J. C., Griffin D. E., Hardwick J. M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993 Feb 25;361(6414):739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Battenberg E. L., Bloom F. E., Oldstone M. B. Viral infection of neurons can depress neurotransmitter mRNA levels without histologic injury. Brain Res. 1988 Jun 7;451(1-2):333–339. doi: 10.1016/0006-8993(88)90779-2. [DOI] [PubMed] [Google Scholar]
- Mahy B. W. Strategies of virus persistence. Br Med Bull. 1985 Jan;41(1):50–55. doi: 10.1093/oxfordjournals.bmb.a072024. [DOI] [PubMed] [Google Scholar]
- Martínez Segovia Z. M., De Mitri M. I., Bendersky S. Nutritional requirements for Junin virus production in BHK culture cells. Acta Physiol Lat Am. 1974;24(6):656–661. [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Viral persistence. Cell. 1989 Feb 24;56(4):517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Viruses can cause disease in the absence of morphological evidence of cell injury: implication for uncovering new diseases in the future. J Infect Dis. 1989 Mar;159(3):384–389. doi: 10.1093/infdis/159.3.384. [DOI] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Banerjee S. N., McMillan C. A., Buchmeier M. J. Inhibition of Pichinde virus replication by actinomycin D. J Gen Virol. 1976 Dec;33(3):421–434. doi: 10.1099/0022-1317-33-3-421. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Buchmeier M. J., Oldstone M. B., Lampert P. W. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Am J Pathol. 1983 Jan;110(1):95–100. [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Price R. W. Early inhibition of acetylcholinesterase and choline acetyltransferase activity in herpes simplex virus type 1 infection of PC12 cells. J Neurochem. 1984 Jan;42(1):142–150. doi: 10.1111/j.1471-4159.1984.tb09710.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein R., Price R. W., Joh T. Alteration of tyrosine hydroxylase activity in PC12 cells infected with herpes simplex virus type 1. Arch Virol. 1985;83(1-2):65–82. doi: 10.1007/BF01310965. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Rukenstein A., Rydel R. E., Greene L. A. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991 Aug;11(8):2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysiecki G., Gewert D. R., Williams B. R. Constitutive expression of a 2',5'-oligoadenylate synthetase cDNA results in increased antiviral activity and growth suppression. J Interferon Res. 1989 Dec;9(6):649–657. doi: 10.1089/jir.1989.9.649. [DOI] [PubMed] [Google Scholar]
- Schwartz R., Löhler J., Lehmann-Grube F. Infection of cultivated mouse peritoneal macrophages with lymphocytic choriomeningitis virus. J Gen Virol. 1978 Jun;39(3):565–570. doi: 10.1099/0022-1317-39-3-565. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Tirone F., Shooter E. M. Early gene regulation by nerve growth factor in PC12 cells: induction of an interferon-related gene. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2088–2092. doi: 10.1073/pnas.86.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille A., Gessner A., Lother H., Lehmann-Grube F. Mechanism of recovery from acute virus infection. VIII. Treatment of lymphocytic choriomeningitis virus-infected mice with anti-interferon-gamma monoclonal antibody blocks generation of virus-specific cytotoxic T lymphocytes and virus elimination. Eur J Immunol. 1989 Jul;19(7):1283–1288. doi: 10.1002/eji.1830190720. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Glaser R., Rapp F. Effect of dibutyryl cyclic AMP on the induction of Epstein-Barr virus in hybrid cells. J Virol. 1973 Dec;12(6):1442–1445. doi: 10.1128/jvi.12.6.1442-1445.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza N. J., Dohadwalla A. N., Reden J. Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev. 1983 Apr-Jun;3(2):201–219. doi: 10.1002/med.2610030205. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Borrow P., Oldstone M. B. Viral persistence and disease: cytopathology in the absence of cytolysis. Br Med Bull. 1991 Oct;47(4):838–851. doi: 10.1093/oxfordjournals.bmb.a072515. [DOI] [PubMed] [Google Scholar]