Abstract
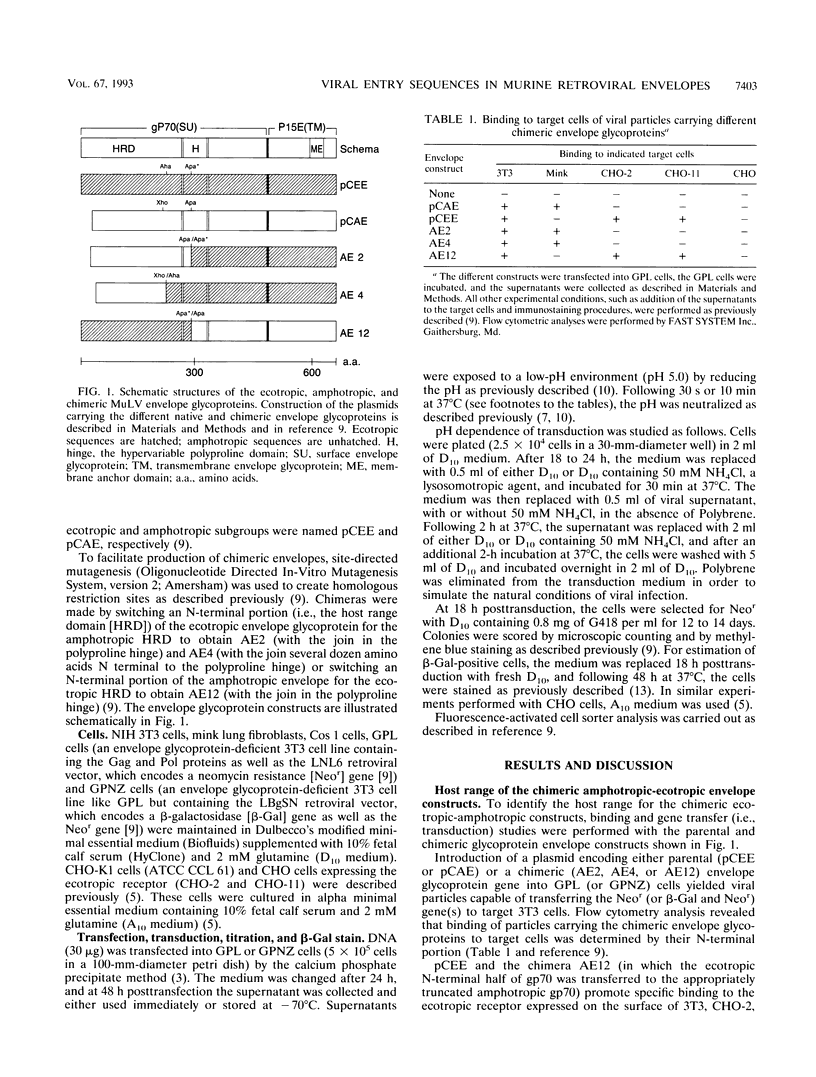
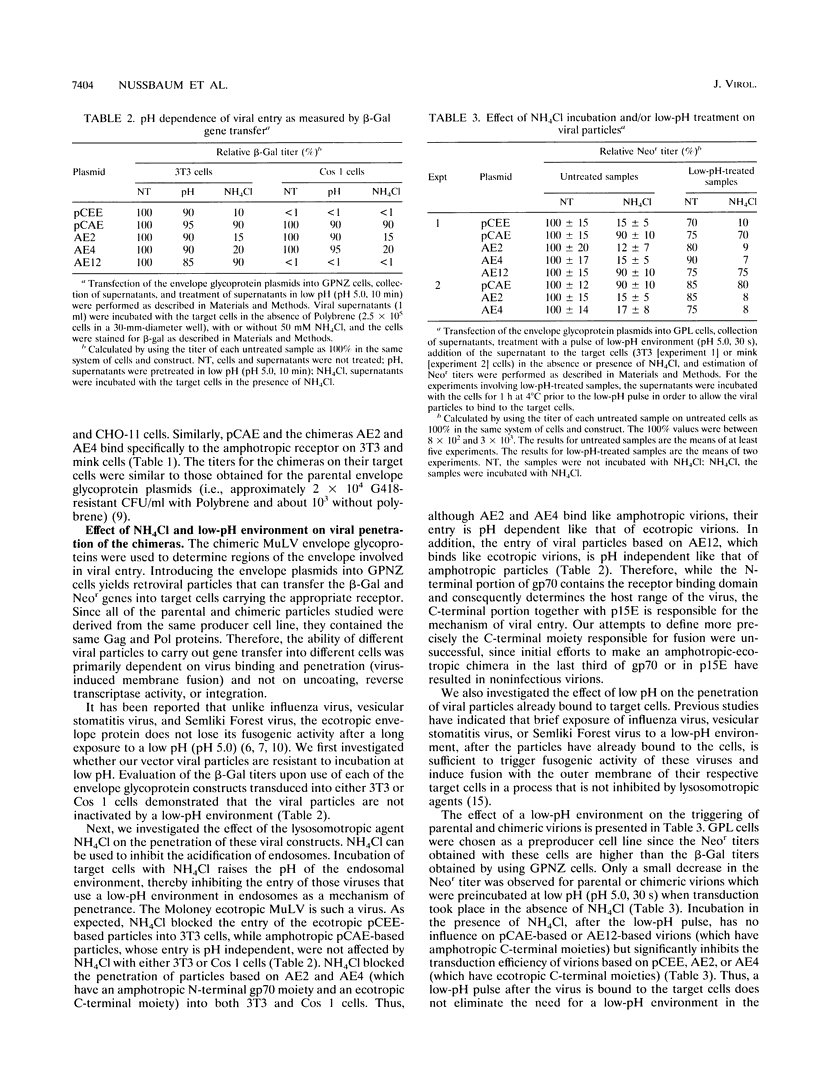
The entry of ecotropic and amphotropic murine leukemia retroviruses (MuLV) into cells was investigated by using viral vector particles carrying chimeric amphotropic-ecotropic envelope glycoproteins on their surface. Chimeras were made by joining, at or near the polyproline hinge, the N-terminal portion of the amphotropic (4070A) gp70 onto the C-terminal portion of the ecotropic (Moloney) gp70 and p15E (constructs AE2 and AE4) or vice versa (AE12). Transduction efficiency of the constructs was tested on target cells that either have only ecotropic receptors (CHO-2 and CHO-11 cells), only amphotropic receptors (mink lung fibroblasts and Cos 1 cells), or both types of receptors (NIH 3T3 cells). The assay made use of the fact that the mechanism for viral entry of ecotropic viruses is pH dependent while that of amphotropic viruses is pH independent. Treatment of target cells with NH4Cl, which prevents the reduction of pH within endosomes, reduced the titers of viral particles bearing the C-terminal moiety from the ecotropic envelope but did not reduce the titers of particles which had a C-terminal moiety from the amphotropic envelope. In addition, in contrast to other low-pH-dependent enveloped viruses, brief acid treatment did not allow surface-bound viruses to bypass the NH4Cl block. The results indicate that the pH dependence of viral entry is a property of the sequences C terminal to the polyproline hinge.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battini J. L., Heard J. M., Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992 Mar;66(3):1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993 Jan;67(1):67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan M. J., Sturm S., Anderson W. F., Eglitis M. A. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J Virol. 1992 Apr;66(4):2281–2287. doi: 10.1128/jvi.66.4.2281-2287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Sommerfelt M. A., Marsh M., Weiss R. A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990 Apr;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Nussbaum O., Muenchau D. D., Shu L., Couture L., Anderson W. F. Analysis of the functional and host range-determining regions of the murine ectropic and amphotropic retrovirus envelope proteins. J Virol. 1993 Aug;67(8):4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O., Loyter A. Quantitative determination of virus-membrane fusion events. Fusion of influenza virions with plasma membranes and membranes of endocytic vesicles in living cultured cells. FEBS Lett. 1987 Aug 31;221(1):61–67. doi: 10.1016/0014-5793(87)80352-6. [DOI] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



