Abstract
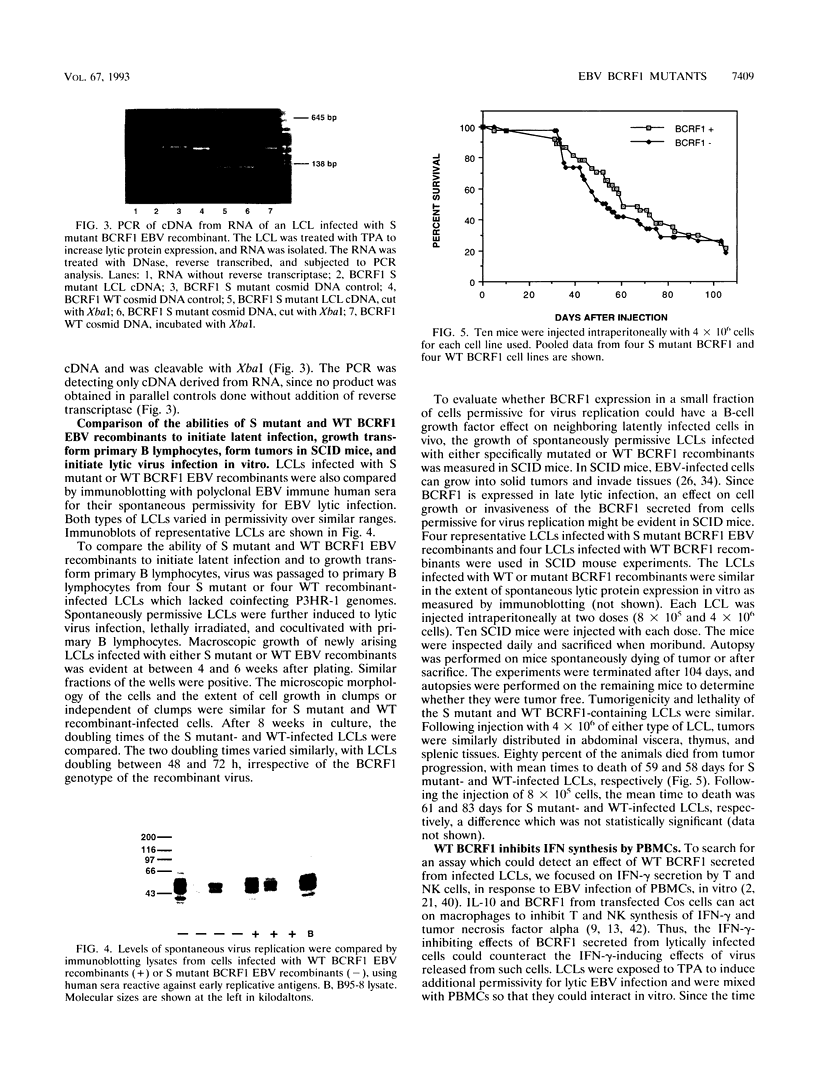
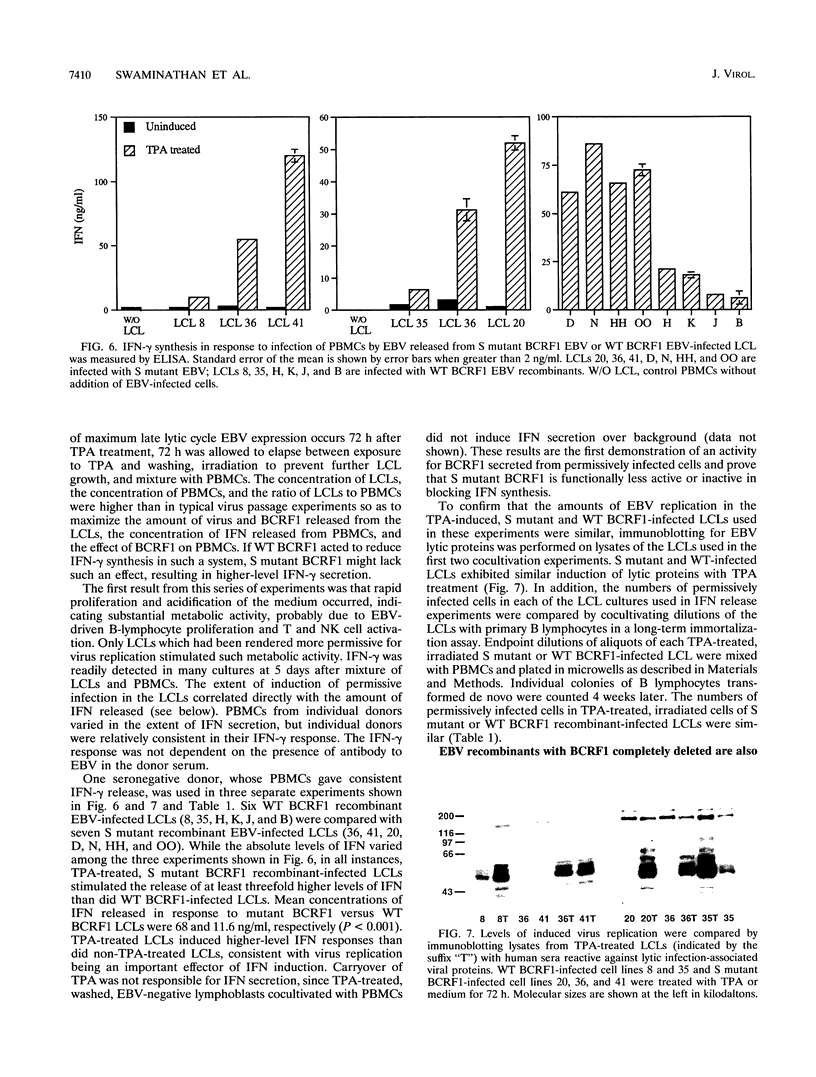
Epstein-Barr virus (EBV) recombinants with specifically mutated BCRF1 genes were constructed and compared with wild-type BCRF1 recombinants derived in parallel for the ability to initiate and maintain latent infection and growth transformation in primary human B lymphocytes. A stop codon insertion after codon 116 of the 170-codon BCRF1 open reading frame or deletion of the entire gene had no effect on latent infection, B-lymphocyte proliferation into long-term lymphoblastoid cell lines (LCLs), or virus replication. LCLs infected with the stop codon recombinant were indistinguishable from wild-type recombinant-infected LCLs in tumorigenicity in SCID mice. However, mutant BCRF1 recombinant-infected cells differed from wild-type recombinant-infected cells in their inability to block gamma interferon release in cultures of permissively infected LCLs incubated with autologous human peripheral blood mononuclear cells. This is the first functional assay for BCRF1 expression from the EBV genome. BCRF1 probably plays a key role in modulating the specific and nonspecific host responses to EBV infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Britton S., De Ley M., Bird G. Evidence for the ontogenic precedence of suppressor T cell functions in the human neonate. Eur J Immunol. 1983 Jan;13(1):6–13. doi: 10.1002/eji.1830130104. [DOI] [PubMed] [Google Scholar]
- Andersson U., Martinez-Maza O., Andersson J., Britton S., Gadler H., De Ley M., Modrow S. Secretion of gamma-interferon at the cellular level. Induction by Epstein-Barr virus. Scand J Immunol. 1984 Nov;20(5):425–432. doi: 10.1111/j.1365-3083.1984.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M., Farrell P. J. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol. 1987 Nov;61(11):3424–3430. doi: 10.1128/jvi.61.11.3424-3430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilie D., Touitou R., Raphael M., Peuchmaur M., Devergnee O., Rea D., Coumbraras J., Crevon M. C., Edelman L., Joab I. In vivo production of interleukin-10 by malignant cells in AIDS lymphomas. Eur J Immunol. 1992 Nov;22(11):2937–2942. doi: 10.1002/eji.1830221127. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Bankier A. T., Satchwell S. C., Barrell B. G. The short unique region of the B95-8 Epstein-Barr virus genome. Virology. 1985 Nov;147(1):81–98. doi: 10.1016/0042-6822(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Kim J. M., Brannan C. I., Copeland N. G., Jenkins N. A., Khan T. A., Moore K. W. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992 Jun 1;148(11):3618–3623. [PubMed] [Google Scholar]
- Kurilla M. G., Swaminathan S., Welsh R. M., Kieff E., Brutkiewicz R. R. Effects of virally expressed interleukin-10 on vaccinia virus infection in mice. J Virol. 1993 Dec;67(12):7623–7628. doi: 10.1128/jvi.67.12.7623-7628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde A., Andersson B., Svenson S. B., Ahrne H., Carlsson M., Forsberg P., Hugo H., Karstorp A., Lenkei R., Lindwall A. Serum levels of lymphokines and soluble cellular receptors in primary Epstein-Barr virus infection and in patients with chronic fatigue syndrome. J Infect Dis. 1992 Jun;165(6):994–1000. doi: 10.1093/infdis/165.6.994. [DOI] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Carson D. A., Vaughan J. H. Regulation of Epstein-Barr virus infection by recombinant interferons. Selected sensitivity to interferon-gamma. Eur J Immunol. 1985 May;15(5):520–525. doi: 10.1002/eji.1830150518. [DOI] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Dinarello C. A., Carson D. A., Vaughan J. H. Release of lymphokines after Epstein Barr virus infection in vitro. I. Sources of and kinetics of production of interferons and interleukins in normal humans. J Immunol. 1986 May 15;136(10):3636–3642. [PubMed] [Google Scholar]
- MacNeil I. A., Suda T., Moore K. W., Mosmann T. R., Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990 Dec 15;145(12):4167–4173. [PubMed] [Google Scholar]
- Mannick J. B., Cohen J. I., Birkenbach M., Marchini A., Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991 Dec;65(12):6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. III. Activation of cytotoxic T cells in virus-infected leukocyte cultures. Int J Cancer. 1979 May 15;23(5):618–625. doi: 10.1002/ijc.2910230506. [DOI] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Wallace L. E., Rowe M., Misko I. S., Epstein M. A., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4216–4221. [PubMed] [Google Scholar]
- Rooney C. M., Brimmell M., Buschle M., Allan G., Farrell P. J., Kolman J. L. Host cell and EBNA-2 regulation of Epstein-Barr virus latent-cycle promoter activity in B lymphocytes. J Virol. 1992 Jan;66(1):496–504. doi: 10.1128/jvi.66.1.496-504.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J., Farley J., Strominger J. L., Fresen K. O., Cho M. S., zur Hausen H. Transformation by Epstein-Barr virus requires DNA sequences in the region of BamHI fragments Y and H. J Virol. 1985 Aug;55(2):286–297. doi: 10.1128/jvi.55.2.286-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99(1):8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- Stewart J. P., Rooney C. M. The interleukin-10 homolog encoded by Epstein-Barr virus enhances the reactivation of virus-specific cytotoxic T cell and HLA-unrestricted killer cell responses. Virology. 1992 Dec;191(2):773–782. doi: 10.1016/0042-6822(92)90253-l. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Huneycutt B. S., Reiss C. S., Kieff E. Epstein-Barr virus-encoded small RNAs (EBERs) do not modulate interferon effects in infected lymphocytes. J Virol. 1992 Aug;66(8):5133–5136. doi: 10.1128/jvi.66.8.5133-5136.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Tomkinson B., Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Tomkinson B. E., Wagner D. K., Nelson D. L., Sullivan J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987 Dec 1;139(11):3802–3807. [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Strominger J. L., Speck S. H. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6498–6502. doi: 10.1073/pnas.86.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R. One-signal requirement for interferon-gamma production by human large granular lymphocytes. J Immunol. 1987 Aug 1;139(3):724–727. [PubMed] [Google Scholar]