Abstract
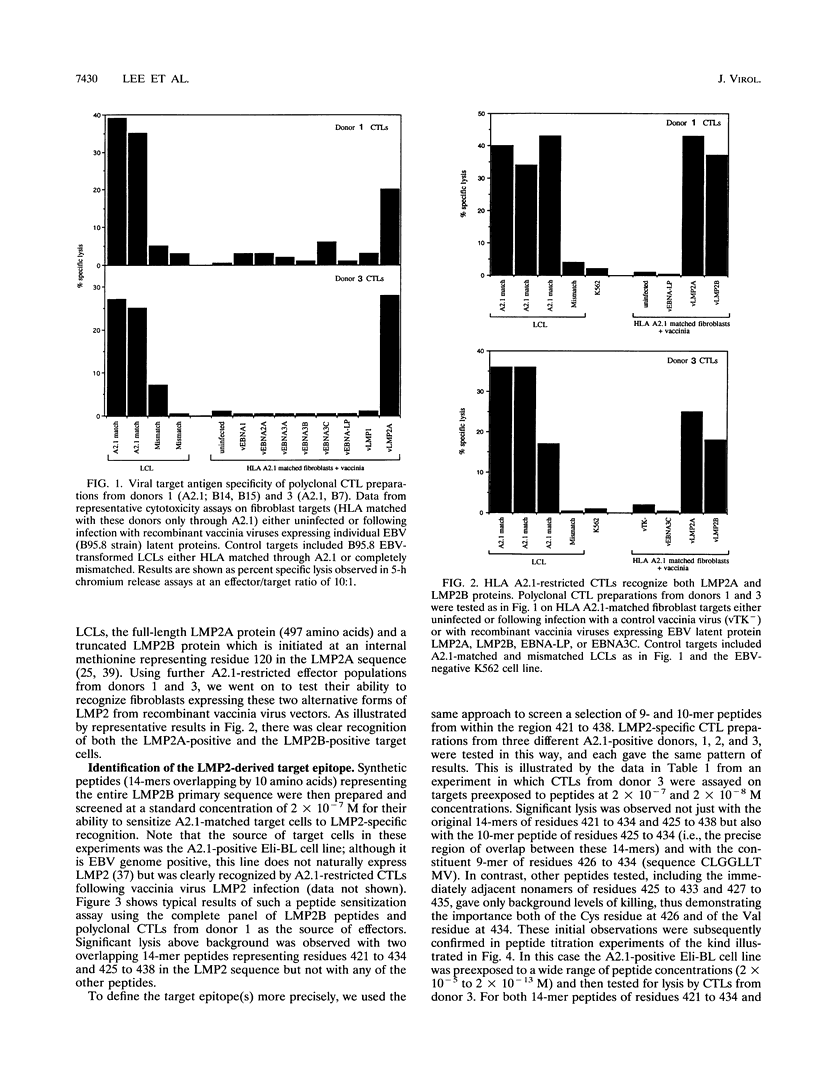
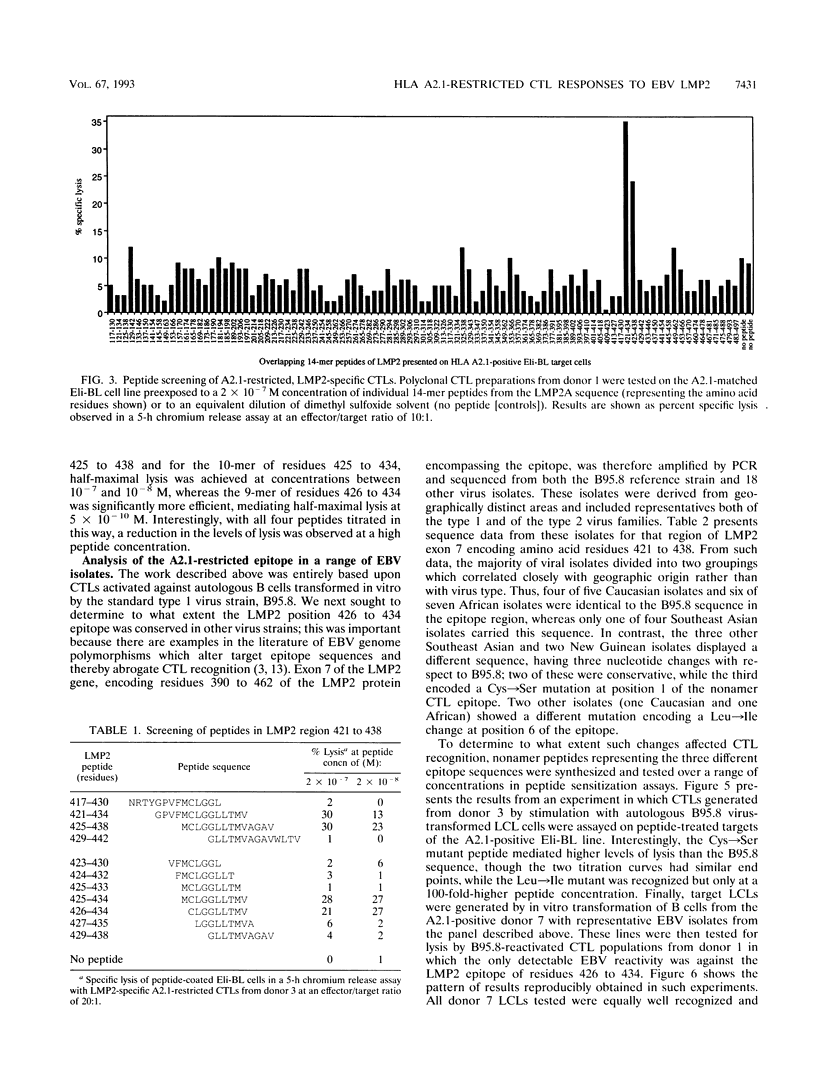
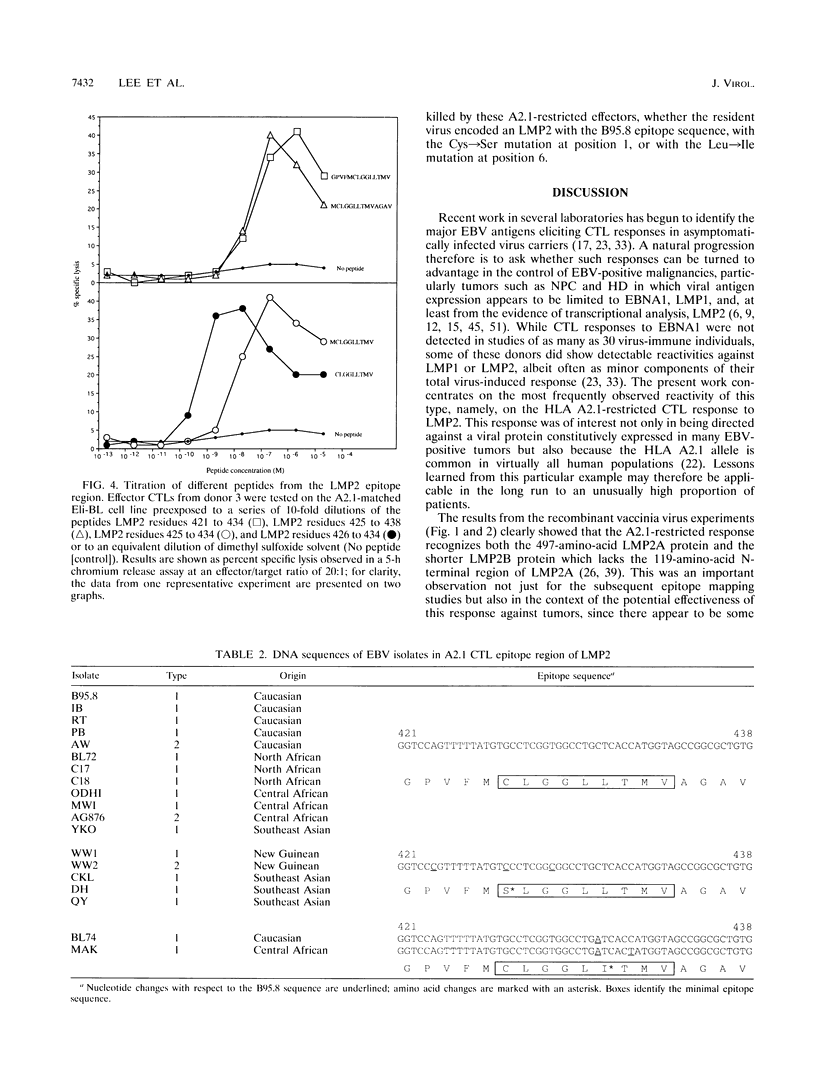
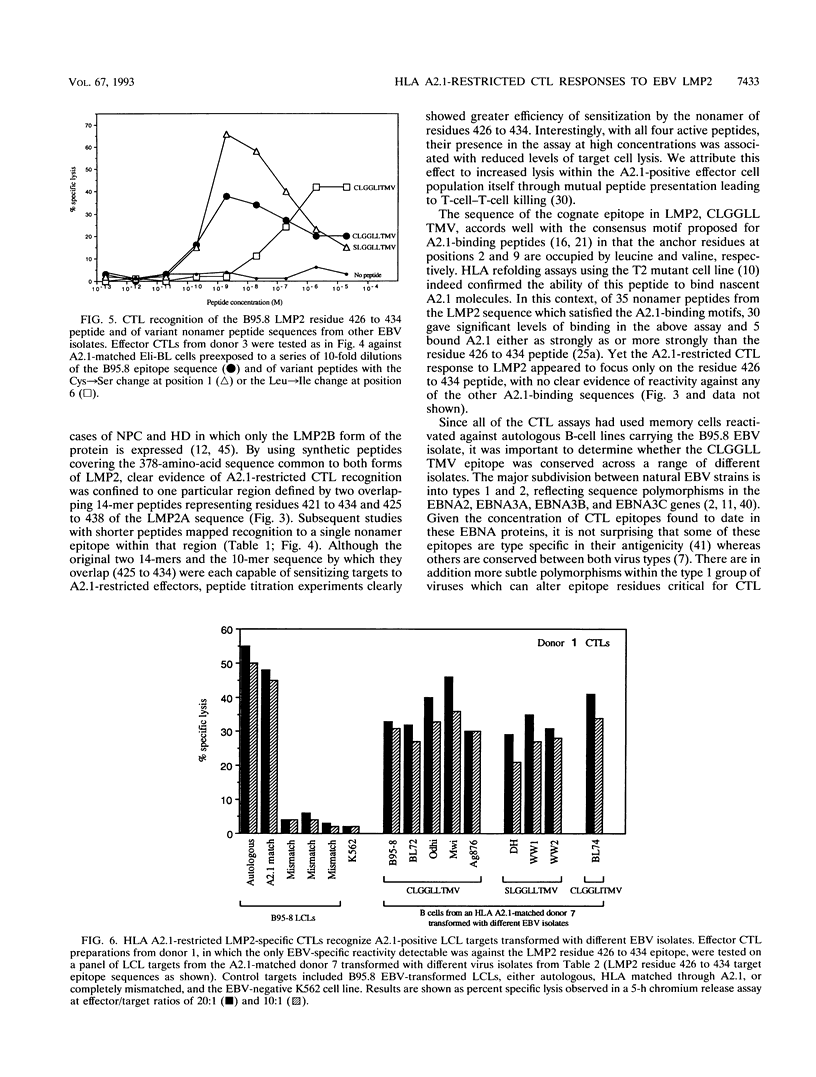
Cytotoxic T-lymphocyte (CTL) responses induced by persistent Epstein-Barr virus (EBV) infection in normal B-lymphoid tissues could potentially be directed against EBV-positive malignancies if expression of the relevant viral target proteins is maintained in tumor cells. For malignancies such as nasopharyngeal carcinoma and Hodgkin's disease, this will require CTL targeting against the nuclear antigen EBNA1 or the latent membrane proteins LMP1 and LMP2. Here we analyze in detail a B95.8 EBV-reactivated CTL response which is specific for LMP2 and restricted through a common HLA allele, A2.1. We found that in vitro-reactivated CTL preparations from several A2.1-positive virus-immune donors contained detectable reactivity against A2.1-bearing target cells expressing either LMP2A or the smaller LMP2B protein from recombinant vaccinia virus vectors. Peptide sensitization experiments then mapped the A2.1-restricted response to a single epitope, the nonamer CLGGLLTMV (LMP2A residues 426 to 434), whose sequence accords well with the proposed peptide binding motif for A2.1. Most Caucasian and African virus isolates (whether of type 1 or type 2) were identical in sequence to B95.8 across this LMP2 epitope region, although 2 of 12 such isolates encoded a Leu-->Ile change at epitope position 6. In contrast, most Southeast Asian and New Guinean isolates (whether of type 1 or type 2) constituted a different virus group with a Cys-->Ser mutation at epitope position 1. CTLs raised against the B95.8-encoded epitope were nevertheless able to recognize these variant epitope sequences in the context of A2.1 whether they were provided exogenously as synthetic peptides or generated endogenously in B cells transformed with the variant viruses. A CTL response of this kind could have therapeutic potential in that it is directed against a protein expressed in many EBV-positive malignancies, is reactive across a range of virus isolates, and is restricted through a relatively common HLA allele.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Hamid M., Chen J. J., Constantine N., Massoud M., Raab-Traub N. EBV strain variation: geographical distribution and relation to disease state. Virology. 1992 Sep;190(1):168–175. doi: 10.1016/0042-6822(92)91202-6. [DOI] [PubMed] [Google Scholar]
- Adldinger H. K., Delius H., Freese U. K., Clarke J., Bornkamm G. W. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology. 1985 Mar;141(2):221–234. doi: 10.1016/0042-6822(85)90253-3. [DOI] [PubMed] [Google Scholar]
- Apolloni A., Moss D., Stumm R., Burrows S., Suhrbier A., Misko I., Schmidt C., Sculley T. Sequence variation of cytotoxic T cell epitopes in different isolates of Epstein-Barr virus. Eur J Immunol. 1992 Jan;22(1):183–189. doi: 10.1002/eji.1830220127. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Brooks J. M., Murray R. J., Thomas W. A., Kurilla M. G., Rickinson A. B. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993 Sep 1;178(3):879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L., Yao Q. Y., Rickinson A. B., Young L. S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992 May;66(5):2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows S. R., Misko I. S., Sculley T. B., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T-cell epitope present on A- and B-type transformants. J Virol. 1990 Aug;64(8):3974–3976. doi: 10.1128/jvi.64.8.3974-3976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows S. R., Rodda S. J., Suhrbier A., Geysen H. M., Moss D. J. The specificity of recognition of a cytotoxic T lymphocyte epitope. Eur J Immunol. 1992 Jan;22(1):191–195. doi: 10.1002/eji.1830220128. [DOI] [PubMed] [Google Scholar]
- Busson P., McCoy R., Sadler R., Gilligan K., Tursz T., Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992 May;66(5):3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon E. M., Pallesen G., Niedobitek G., Crocker J., Brooks L., Rickinson A. B., Young L. S. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993 Feb 1;177(2):339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas B. T. Standards for total serum protein assays--a collaborative study. Clin Chem. 1975 Jul;21(8):1159–1166. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Gavioli R., De Campos-Lima P. O., Kurilla M. G., Kieff E., Klein G., Masucci M. G. Recognition of the Epstein-Barr virus-encoded nuclear antigens EBNA-4 and EBNA-6 by HLA-A11-restricted cytotoxic T lymphocytes: implications for down-regulation of HLA-A11 in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5862–5866. doi: 10.1073/pnas.89.13.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli R., Kurilla M. G., de Campos-Lima P. O., Wallace L. E., Dolcetti R., Murray R. J., Rickinson A. B., Masucci M. G. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol. 1993 Mar;67(3):1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Zwaan F. E., Lepoutre J., Klein G., Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8693–8696. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Zutter M. M., Minarovits J., Oosterveer M. A., Thomas E. D., Klein G., Ernberg I. Expression of Epstein-Barr virus-encoded growth-transformation-associated proteins in lymphoproliferations of bone-marrow transplant recipients. Int J Cancer. 1991 Jan 21;47(2):188–192. doi: 10.1002/ijc.2910470205. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Perricaudet M., Farrell P. J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988 Mar;7(3):769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990 May;64(5):2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. J., Day N. E., Degos L., Lepage V., Wang P. C., Chan S. H., Simons M., McKnight B., Easton D., Zeng Y. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature. 1990 Aug 2;346(6283):470–471. doi: 10.1038/346470a0. [DOI] [PubMed] [Google Scholar]
- Lung M. L., Lam W. P., Sham J., Choy D., Yong-Sheng Z., Guo H. Y., Ng M. H. Detection and prevalence of the "f" variant of Epstein-Barr virus in southern China. Virology. 1991 Nov;185(1):67–71. doi: 10.1016/0042-6822(91)90754-y. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Burrows S. R., Baxter G. D., Lavin M. F. T cell-T cell killing is induced by specific epitopes: evidence for an apoptotic mechanism. J Exp Med. 1991 Mar 1;173(3):681–686. doi: 10.1084/jem.173.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Burrows S. R., Khanna R., Misko I. S., Sculley T. B. Immune surveillance against Epstein-Barr virus. Semin Immunol. 1992 Apr;4(2):97–104. [PubMed] [Google Scholar]
- Moss D. J., Misko I. S., Burrows S. R., Burman K., McCarthy R., Sculley T. B. Cytotoxic T-cell clones discriminate between A- and B-type Epstein-Barr virus transformants. Nature. 1988 Feb 25;331(6158):719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Murray R. J., Brooks J., Griffin H., Moss D. J., Masucci M. G. T cell recognition of Epstein-Barr virus associated lymphomas. Cancer Surv. 1992;13:53–80. [PubMed] [Google Scholar]
- Riddell S. R., Watanabe K. S., Goodrich J. M., Li C. R., Agha M. E., Greenberg P. D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Rooney C. M., Gregory C. D., Rowe M., Finerty S., Edwards C., Rupani H., Rickinson A. B. Endemic Burkitt's lymphoma: phenotypic analysis of tumor biopsy cells and of derived tumor cell lines. J Natl Cancer Inst. 1986 Sep;77(3):681–687. doi: 10.1093/jnci/77.3.681. [DOI] [PubMed] [Google Scholar]
- Rowe M., Lear A. L., Croom-Carter D., Davies A. H., Rickinson A. B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992 Jan;66(1):122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Liebowitz D., Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989 Feb;63(2):933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Burrows S. R., Sculley T. B., Moss D. J., Misko I. S. Nonresponsiveness to an immunodominant Epstein-Barr virus-encoded cytotoxic T-lymphocyte epitope in nuclear antigen 3A: implications for vaccine strategies. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9478–9482. doi: 10.1073/pnas.88.21.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R. S., McClain K., Frizzera G., Gajl-Peczalska K. J., Kersey J. H., Blazar B. R., Arthur D. C., Patton D. F., Greenberg J. S., Burke B. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988 May;71(5):1234–1243. [PubMed] [Google Scholar]
- Simons M. J., Wee G. B., Chan S. H., Shanmugaratnam K. Probable identification of an HL-A second-locus antigen associated with a high risk of nasopharyngeal carcinoma. Lancet. 1975 Jan 18;1(7899):142–143. doi: 10.1016/s0140-6736(75)91433-6. [DOI] [PubMed] [Google Scholar]
- Simons M. J., Wee G. B., Day N. E., Morris P. J., Shanmugaratnam K., De-Thé G. B. Immunogenetic aspects of nasopharyngeal carcinoma: I. Differences in HL-A antigen profiles between patients and control groups. Int J Cancer. 1974 Jan 15;13(1):122–134. doi: 10.1002/ijc.2910130114. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Griffin B. E. Differential expression of Epstein Barr viral transcripts for two proteins (TP1 and LMP) in lymphocyte and epithelial cells. Nucleic Acids Res. 1991 May 11;19(9):2435–2440. doi: 10.1093/nar/19.9.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr, Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Wallace L. E., Rowe M., Gaston J. S., Rickinson A. B., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. III. Establishment of HLA-restricted cytotoxic T cell lines using interleukin 2. Eur J Immunol. 1982 Dec;12(12):1012–1018. doi: 10.1002/eji.1830121206. [DOI] [PubMed] [Google Scholar]
- Yao Q. Y., Ogan P., Rowe M., Wood M., Rickinson A. B. Epstein-Barr virus-infected B cells persist in the circulation of acyclovir-treated virus carriers. Int J Cancer. 1989 Jan 15;43(1):67–71. doi: 10.1002/ijc.2910430115. [DOI] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- Zutter M. M., Martin P. J., Sale G. E., Shulman H. M., Fisher L., Thomas E. D., Durnam D. M. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988 Aug;72(2):520–529. [PubMed] [Google Scholar]
- de Campos-Lima P. O., Gavioli R., Zhang Q. J., Wallace L. E., Dolcetti R., Rowe M., Rickinson A. B., Masucci M. G. HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+ population. Science. 1993 Apr 2;260(5104):98–100. doi: 10.1126/science.7682013. [DOI] [PubMed] [Google Scholar]



