Abstract
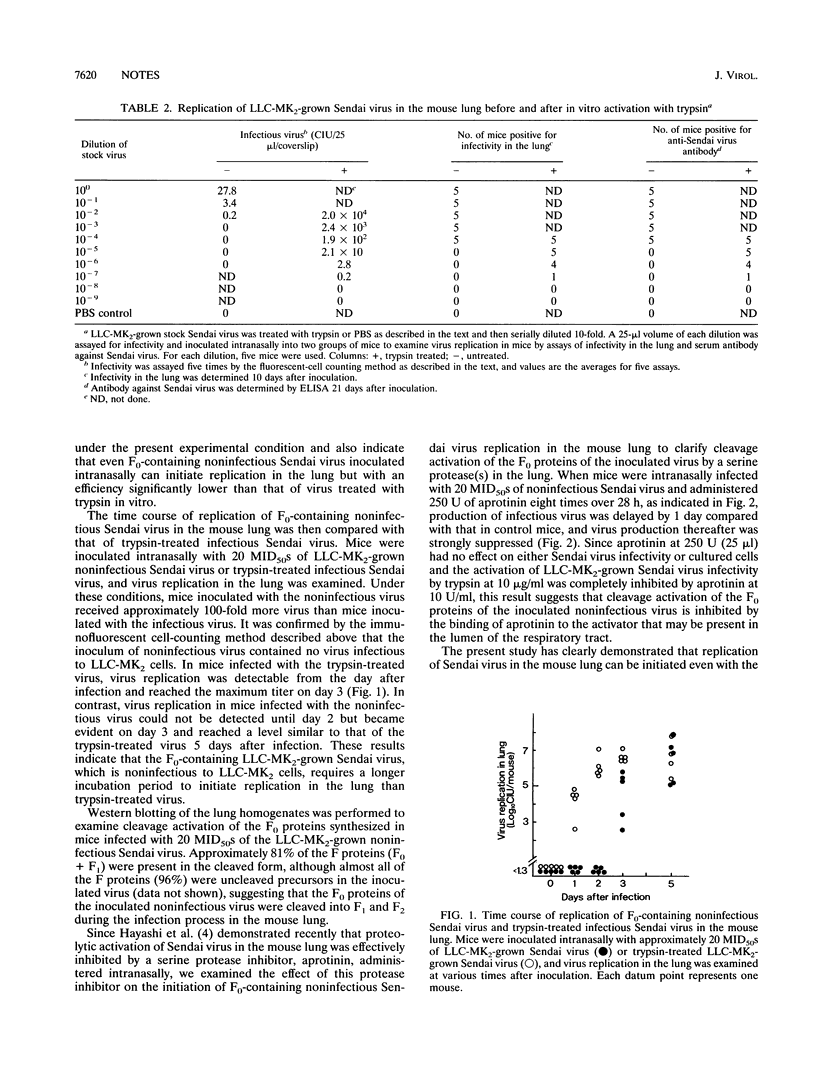
The replication of LLC-MK2-grown noninfectious Sendai virus, containing exclusively fusion (F) glycoprotein precursors, was examined in the mouse lung to study the accessibility of virus inoculated intranasally to the virus activator present in the lung. When mice were intranasally inoculated with various doses of the virus after in vitro activation with trypsin, the 50% mouse infectious dose (MID50) was determined to be 0.7 cell-infectious units (CIU) per mouse, indicating that one infectious unit of Sendai virus is enough to initiate replication in the mouse lung and that the present experimental system is highly sensitive. On the other hand, in mice inoculated with virus not treated with trypsin, virus replication in the lung was recognized even in mice inoculated with samples containing no infectious virus, and the MID50 was determined to be 67.5 CIU per mouse (here, CIU were assayed after in vitro trypsin treatment). When mice were infected with 20 MID50 of trypsin-treated infectious and untreated noninfectious viruses (an approximately 100-fold greater amount of noninfectious virus than of infectious virus was used), the noninfectious virus was found to require 2 more days of incubation than the infectious virus, and many of the F proteins synthesized in the lungs of mice infected with the F0-containing virus were present in the cleaved form. In addition, the infection of mice with noninfectious virus was strongly suppressed by aprotinin, a serine protease inhibitor. These results indicate that Sendai virus can initiate replication in the mouse lung even with the F0-containing noninfectious virus and strongly suggest that this infection process is mediated by cleavage activation of the F0 proteins of inoculated viruses by a serine protease(s) present in the lumen of the mouse respiratory tract but that activation of the noninfectious virus is an inefficient process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Gotoh B., Ogasawara T., Toyoda T., Inocencio N. M., Hamaguchi M., Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990 Dec;9(12):4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Hotta H., Itoh M., Homma M. Protection of mice by a protease inhibitor, aprotinin, against lethal Sendai virus pneumonia. J Gen Virol. 1991 Apr;72(Pt 4):979–982. doi: 10.1099/0022-1317-72-4-979. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Choppin P. W. Analysis of Sendai virus mRNAs with cDNA clones of viral genes and sequences of biologically important regions of the fusion protein. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7732–7736. doi: 10.1073/pnas.81.24.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Homma M. Sendai virus. Adv Virus Res. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- Ito Y., Yamamoto F., Takano M., Maeno K., Shimokata K., Iinuma M., Hara K., Iijima S. Detection of cellular receptors for Sendai virus in mouse tissue sections. Arch Virol. 1983;75(1-2):103–113. doi: 10.1007/BF01314130. [DOI] [PubMed] [Google Scholar]
- Kido H., Yokogoshi Y., Sakai K., Tashiro M., Kishino Y., Fukutomi A., Katunuma N. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biol Chem. 1992 Jul 5;267(19):13573–13579. [PubMed] [Google Scholar]
- Kiyotani K., Takao S., Sakaguchi T., Yoshida T. Immediate protection of mice from lethal wild-type Sendai virus (HVJ) infections by a temperature-sensitive mutant, HVJpi, possessing homologous interfering capacity. Virology. 1990 Jul;177(1):65–74. doi: 10.1016/0042-6822(90)90460-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Isomura S., Suzuki S., Watanabe E., Hamaguchi M., Yoshida T., Nagai Y. Monoclonal antibodies to the HN glycoprotein of Newcastle disease virus. Biological characterization and use for strain comparisons. Virology. 1983 Oct 30;130(2):318–330. doi: 10.1016/0042-6822(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Ogasawara T., Gotoh B., Suzuki H., Asaka J., Shimokata K., Rott R., Nagai Y. Expression of factor X and its significance for the determination of paramyxovirus tropism in the chick embryo. EMBO J. 1992 Feb;11(2):467–472. doi: 10.1002/j.1460-2075.1992.tb05076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Matsuda Y., Kiyokage R., Kawahara N., Kiyotani K., Katunuma N., Nagai Y., Yoshida T. Identification of endoprotease activity in the trans Golgi membranes of rat liver cells that specifically processes in vitro the fusion glycoprotein precursor of virulent Newcastle disease virus. Virology. 1991 Oct;184(2):504–512. doi: 10.1016/0042-6822(91)90420-g. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983 Feb;39(2):879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Takeda M., Tanaka S., Nishimura N., Takenaka M., Kido H. Antibody against the carboxyl terminus of the F2 subunit of Sendai virus fusion glycoprotein inhibits proteolytic activation. Virology. 1993 Jun;194(2):882–885. doi: 10.1006/viro.1993.1336. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Yamakawa M., Tobita K., Klenk H. D., Rott R., Seto J. T. Organ tropism of Sendai virus in mice: proteolytic activation of the fusion glycoprotein in mouse organs and budding site at the bronchial epithelium. J Virol. 1990 Aug;64(8):3627–3634. doi: 10.1128/jvi.64.8.3627-3634.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Yokogoshi Y., Tobita K., Seto J. T., Rott R., Kido H. Tryptase Clara, an activating protease for Sendai virus in rat lungs, is involved in pneumopathogenicity. J Virol. 1992 Dec;66(12):7211–7216. doi: 10.1128/jvi.66.12.7211-7216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Komatsu H., Ohkata K., Nakajima T., Watanabe M., Tanaka Y., Arifuku M. Neutralizing activity of the antibodies against two kinds of envelope glycoproteins of Sendai virus. Arch Virol. 1986;91(1-2):145–161. doi: 10.1007/BF01316735. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Hamaguchi M., Naruse H., Nagai Y. Persistent infection by a temperature-sensitive mutant isolated from a Sendai virus (HVJ) carrier culture: its initiation and maintenance without aid of defective interfering particles. Virology. 1982 Jul 30;120(2):329–339. doi: 10.1016/0042-6822(82)90034-4. [DOI] [PubMed] [Google Scholar]



