Abstract
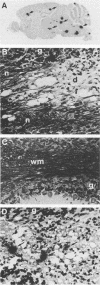
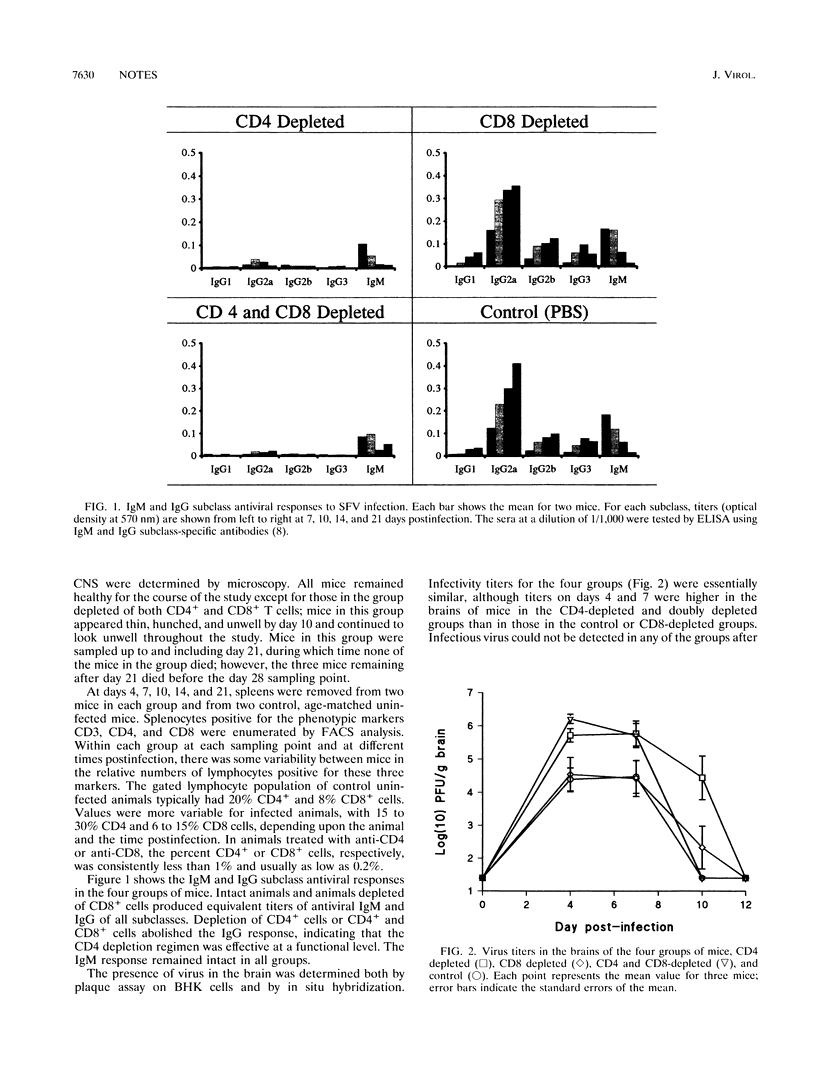
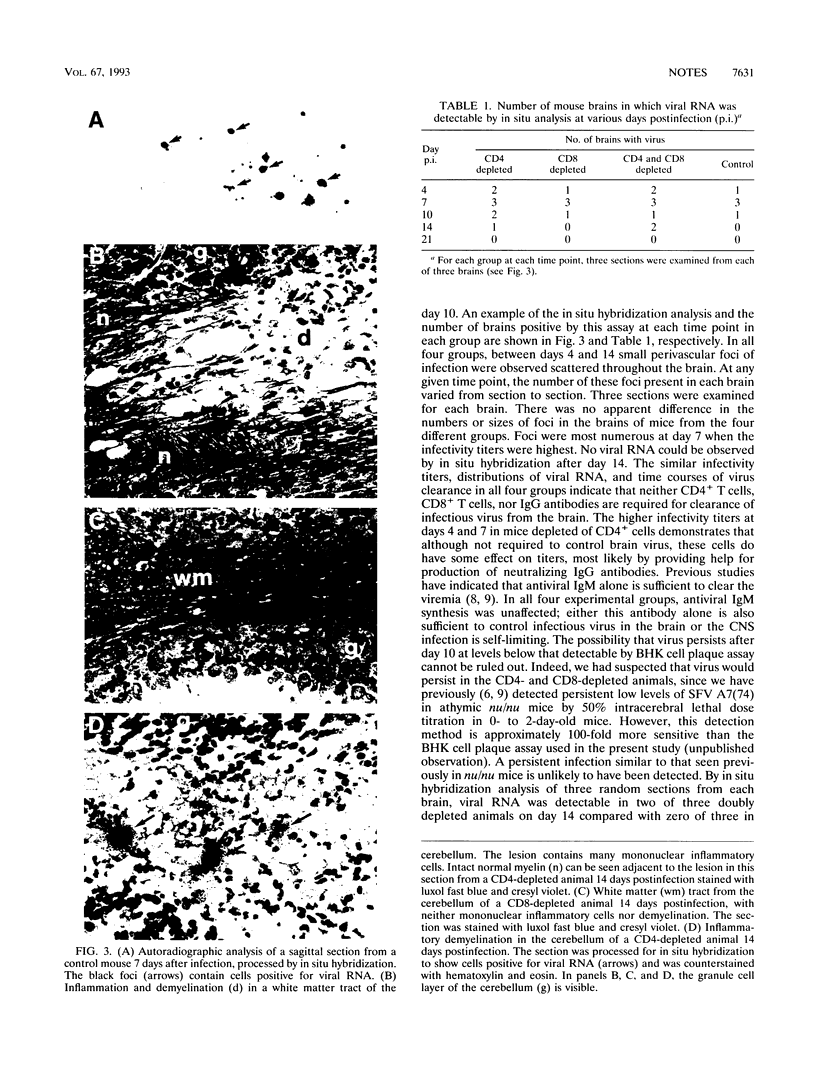
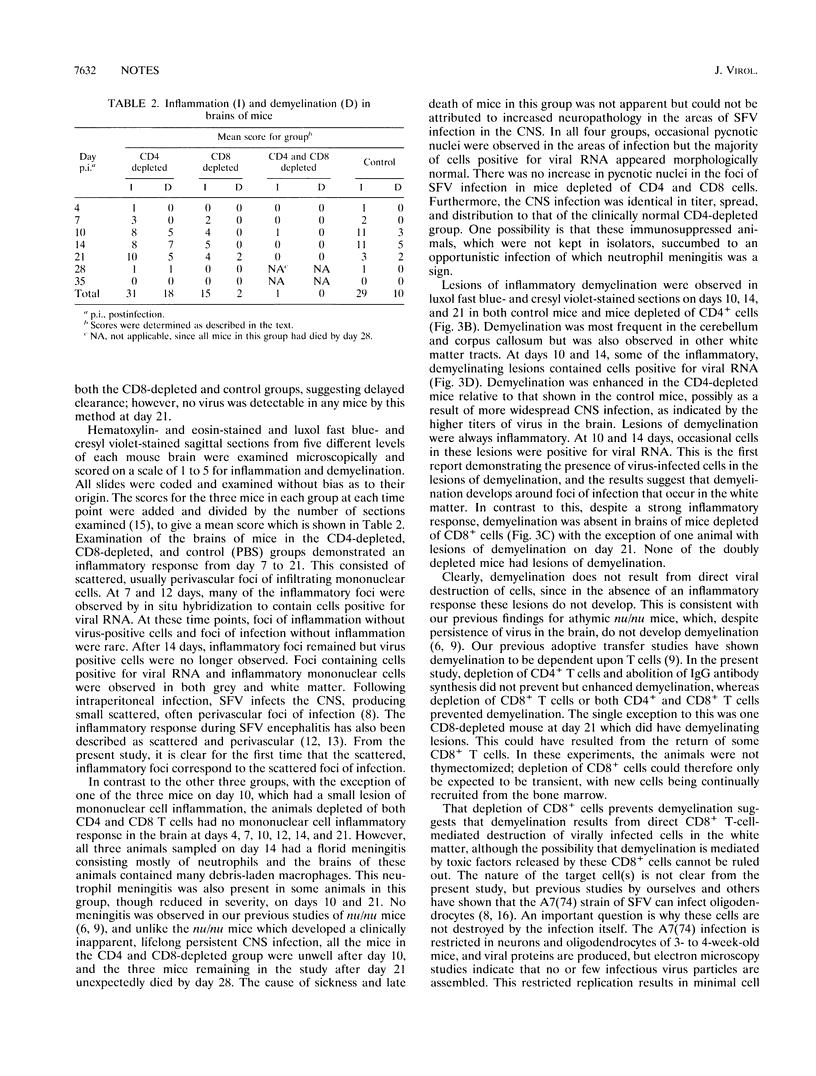
Following intraperitoneal infection of BALB/c mice with the A7(74) strain of Semliki Forest virus, the virus spreads to the central nervous system (CNS) and initiates an acute inflammatory reaction which includes lesions of primary demyelination. This demyelination is dependent upon activated T lymphocytes. To determine whether CD4+ or CD8+ T cells are involved in the pathogenesis of the demyelination, we have investigated the course of infection in animals treated with monoclonal anti-CD4 or anti-CD8 antibodies. In the normal course of infection, virus was detectable in the brain by infectivity assay and in situ hybridization for up to 14 days. Antiviral immunoglobulin M (IgM) and all subclasses of IgG were produced. From day 10 to 21 postinfection lesions of inflammatory demyelination were present, most notably in the cerebellum and corpus callosum but also in other white matter tracts. Administration of anti-CD4 antibodies removed CD4+ cells from the spleen, prevented production of antiviral IgG, increased virus titers in the brain, and increased demyelination. Administration of anti-CD8 antibodies depleted CD8+ cells from the spleen and did not affect antiviral IgG synthesis or spread of brain virus but reduced CNS inflammatory responses and virtually abolished lesions of demyelination. Administration of both antibodies depleted both T-cell subsets from the spleen, prevented IgG antibody production, increased brain virus, and abrogated both CNS inflammation and lesions of demyelination. In conclusion, the CNS demyelination induced by Semliki Forest virus can be prevented by in vivo depletion of CD8+ T lymphocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor S., Webb H. E. The effect of cycloleucine on SFV A7(74) infection in mice. Br J Exp Pathol. 1987 Apr;68(2):225–235. [PMC free article] [PubMed] [Google Scholar]
- Blackman M. J., Morris A. G. Gamma interferon production and cytotoxicity of spleen cells from mice infected with Semliki Forest virus. J Gen Virol. 1984 May;65(Pt 5):955–961. doi: 10.1099/0022-1317-65-5-955. [DOI] [PubMed] [Google Scholar]
- Borrow P., Tonks P., Welsh C. J., Nash A. A. The role of CD8+T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992 Jul;73(Pt 7):1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Calder V. L., Wolswijk G., Noble M. The differentiation of O-2A progenitor cells into oligodendrocytes is associated with a loss of inducibility of Ia antigens. Eur J Immunol. 1988 Aug;18(8):1195–1201. doi: 10.1002/eji.1830180808. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Fazakerley J. K., Amor S., Webb H. E. Reconstitution of Semliki forest virus infected mice, induces immune mediated pathological changes in the CNS. Clin Exp Immunol. 1983 Apr;52(1):115–120. [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Buchmeier M. J. Pathogenesis of virus-induced demyelination. Adv Virus Res. 1993;42:249–324. doi: 10.1016/S0065-3527(08)60087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Pathak S., Scallan M., Amor S., Dyson H. Replication of the A7(74) strain of Semliki Forest virus is restricted in neurons. Virology. 1993 Aug;195(2):627–637. doi: 10.1006/viro.1993.1414. [DOI] [PubMed] [Google Scholar]
- Fazakerley J. K., Webb H. E. Semliki Forest virus induced, immune mediated demyelination: the effect of irradiation. Br J Exp Pathol. 1987 Feb;68(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Webb H. E. Semliki Forest virus-induced, immune-mediated demyelination: adoptive transfer studies and viral persistence in nude mice. J Gen Virol. 1987 Feb;68(Pt 2):377–385. doi: 10.1099/0022-1317-68-2-377. [DOI] [PubMed] [Google Scholar]
- Jenkins H. G., Tansey E. M., Macefield F., Ikeda H. Evidence for a T-cell related factor as the cause of demyelination in mice following Semliki Forest virus infection. Brain Res. 1988 Aug 30;459(1):145–147. doi: 10.1016/0006-8993(88)90294-6. [DOI] [PubMed] [Google Scholar]
- Kelly W. R., Blakemore W. F., Jagelman S., Webb H. E. Demyelination induced in mice by avirulent Semliki Forest virus. II. An ultrastructural study of focal demyelination in the brain. Neuropathol Appl Neurobiol. 1982 Jan-Feb;8(1):43–53. doi: 10.1111/j.1365-2990.1982.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie A., Suckling A. J., Jagelman S., Wilson A. M. Histopathological and enzyme histochemical changes in experimental Semliki Forest virus infection in mice and their relevance to scrapie. J Comp Pathol. 1978 Jul;88(3):335–344. doi: 10.1016/0021-9975(78)90038-5. [DOI] [PubMed] [Google Scholar]
- Parsons L. M., Webb H. E. Blood brain barrier disturbance and immunoglobulin G levels in the cerebrospinal fluid of the mouse following peripheral infection with the demyelinating strain of Semliki Forest virus. J Neurol Sci. 1982 Dec;57(2-3):307–318. doi: 10.1016/0022-510x(82)90037-5. [DOI] [PubMed] [Google Scholar]
- Parsons L. M., Webb H. E. Virus titres and persistently raised white cell counts in cerebrospinal fluid in mice after peripheral infection with demyelinating Semliki Forest virus. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):395–401. doi: 10.1111/j.1365-2990.1982.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Pathak S., Illavia S. J., Webb H. E. The identification and role of cells involved in CNS demyelination in mice after Semliki Forest virus infection: an ultrastructural study. Prog Brain Res. 1983;59:237–254. doi: 10.1016/S0079-6123(08)63869-8. [DOI] [PubMed] [Google Scholar]
- Pathak S., Webb H. E. An electron-microscopic study of avirulent and virulent Semliki forest virus in the brains of different ages of mice. J Neurol Sci. 1978 Dec;39(2-3):199–211. doi: 10.1016/0022-510x(78)90123-5. [DOI] [PubMed] [Google Scholar]
- Pathak S., Webb H. E., Oaten S. W., Bateman S. An electron-microscopic study of the development of virulent and avirulent strains of Semliki forest virus in mouse brain. J Neurol Sci. 1976 Jul;28(3):289–300. doi: 10.1016/0022-510x(76)90022-8. [DOI] [PubMed] [Google Scholar]
- Pathak S., Webb H. E. Possible mechanisms for the transport of Semliki forest virus into and within mouse brain. An electron-microscopic study. J Neurol Sci. 1974 Oct;23(2):175–184. doi: 10.1016/0022-510x(74)90221-4. [DOI] [PubMed] [Google Scholar]
- Pusztai R., Gould E. A., Smith H. Infection patterns in mice of an avirulent and virulent strain of Semliki Forest virus. Br J Exp Pathol. 1971 Dec;52(6):669–677. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Lindsley M. D., Pierce M. L. Role of T cells in resistance to Theiler's virus infection. Microb Pathog. 1991 Oct;11(4):269–281. doi: 10.1016/0882-4010(91)90031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Suckling A. J., Jagelman S., Webb H. E. Brain lysosomal glycosidase activity in immunosuppressed mice infected with avirulent Semliki forest virus. Infect Immun. 1977 Feb;15(2):386–391. doi: 10.1128/iai.15.2.386-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey E. M., Ikeda H. The relationship between axonal transport of protein and demyelination in the optic nerves of mice infected with Semliki Forest virus. Brain Res. 1986 Nov 5;397(1):9–15. doi: 10.1016/0006-8993(86)91364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremain K. E., Ikeda H. Physiological deficits in the visual system of mice infected with Semliki Forest virus and their correlation with those seen in patients with demyelinating disease. Brain. 1983 Dec;106(Pt 4):879–895. doi: 10.1093/brain/106.4.879. [DOI] [PubMed] [Google Scholar]
- Welsh C. J., Tonks P., Nash A. A., Blakemore W. F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987 Jun;68(Pt 6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]