Abstract
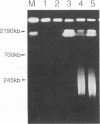
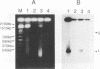
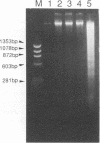
Pulsed-field agarose gel electrophoresis showed that fragmentation of chromosomal DNA in Raji cells was induced by infection with the P3HR-1 strain of Epstein-Barr virus (EBV). S1 nuclease treatment of the agarose plugs containing cells suggested that the majority of DNA fragments did not contain single-strand gaps. Chromosomal DNA fragmentation was inhibited by cycloheximide, indicating that protein synthesis was required for DNA fragmentation. Phosphonoacetic acid, an inhibitor of EBV DNA polymerase, did not inhibit fragmentation of chromosomal DNA. These findings suggest that EBV-specific early proteins participate in fragmentation of chromosomal DNA. Chromosomal DNA of P3HR-1 cells was also fragmented by treatment with n-butyrate plus 12-O-tetradecanoylphorbol-13-acetate (TPA), which induced activation of latent EBV genome following viral replication. In addition, fragmentation of DNA preceded cell death during lytic infection. These results suggest that fragmentation of chromosomal DNA is generally induced during EBV replication and probably contributes to the cytopathic effect of EBV. The role of DNA fragmentation in death of infected cells is discussed in relation to apoptosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Biggin M., Bodescot M., Perricaudet M., Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987 Oct;61(10):3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. Various forms of Epstein-Barr virus infection in man: established facts and a general concept. Lancet. 1973 Oct 13;2(7833):836–839. doi: 10.1016/s0140-6736(73)90874-x. [DOI] [PubMed] [Google Scholar]
- Finke J., Fritzen R., Ternes P., Trivedi P., Bross K. J., Lange W., Mertelsmann R., Dölken G. Expression of bcl-2 in Burkitt's lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992 Jul 15;80(2):459–469. [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced polypeptides: a comparative study with superinfected Raji, IUdR-Treated, and N-butyrate-treated P3HR-1 cells. Virology. 1981 Feb;109(1):72–81. doi: 10.1016/0042-6822(81)90472-4. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. "Thymineless" death in androgen-independent prostatic cancer cells. Biochem Biophys Res Commun. 1989 Nov 30;165(1):73–81. doi: 10.1016/0006-291x(89)91035-8. [DOI] [PubMed] [Google Scholar]
- Laurent-Crawford A. G., Krust B., Muller S., Rivière Y., Rey-Cuillé M. A., Béchet J. M., Montagnier L., Hovanessian A. G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991 Dec;185(2):829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- Long B. H., Stringfellow D. A. Inhibitors of topoisomerase II: structure-activity relationships and mechanism of action of podophyllin congeners. Adv Enzyme Regul. 1988;27:223–256. doi: 10.1016/0065-2571(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai C., Kornbluth R. S., Pauza C. D., Richman D. D., Carson D. A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991 May;87(5):1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- White E., Cipriani R., Sabbatini P., Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991 Jun;65(6):2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]