Abstract
The ability to identify, isolate, and transplant progenitor cells from solid tissues would greatly facilitate the treatment of diseases currently requiring whole organ transplantation. In this study, cell fractions enriched in candidate epithelial progenitor cells from the rat pancreas were isolated and transplanted into the liver of an inbred strain of Fischer rats. Using a dipeptidyl dipeptidase IV genetic marker system to follow the fate of transplanted cells in conjunction with albumin gene expression, we provide conclusive evidence that, after transplantation to the liver, epithelial progenitor cells from the pancreas differentiate into hepatocytes, express liver-specific proteins, and become fully integrated into the liver parenchymal structure. These studies demonstrate the presence of multipotent progenitor cells in the adult pancreas and establish a role for the liver microenvironment in the terminal differentiation of epithelial cells of foregut origin. They further suggest that such progenitor cells might be useful in studies of organ repopulation following acute or chronic liver injury.
The liver and pancreas have a similar structural organization and common embryologic origin (1–4). To initiate development of these organs, epithelial cells of the ventral foregut migrate into the transverse and splanchnic mesoderm, respectively. In the rat, the liver bud first becomes apparent at embryonic day 10 (E10), followed within 24 hr (E11) by the pancreatic bud. In both instances, a rudimentary lobular structure with parenchymal cells draining into ducts is formed by E12 and becomes well developed by E15 in the liver and E16 in the pancreas. During later stages of parenchymal cell maturation (perinatal period), the differentiated function of these organs becomes firmly established through tissue or cell-type specific gene expression programs.
The presence of progenitor cells in the adult liver was originally postulated by Wilson and Leduc (5). Although the liver regenerates following partial hepatectomy by proliferation of mature hepatocytes, recent evidence suggests that, under specialized circumstances, immature epithelial cells can also proliferate and differentiate along the hepatocyte lineage to restore lost hepatic mass (6–9). Thus, these cells can be defined as facultative hepatocyte progenitor cells (for reviews see refs. 10–13).
In the adult rat, under certain pathologic circumstances, such as induction of pancreatic acinar atrophy by dietary copper (Cu) depletion (14, 15), epithelial cells in the pancreas proliferate and express liver-specific genes. Under these conditions, Reddy and coworkers (14, 15) concluded that pancreatic ductal epithelial cells transdifferentiate into hepatocytes. We have used the Cu-depletion/repletion model to show that putative pancreatic epithelial progenitor cells proliferate and begin to express a liver-specific phenotype but do not complete the liver differentiation program normally observed during fetal development (16). Genes expressed in the early hepatoblast, such as α-fetoprotein and albumin, are induced, as well as genes expressed later during fetal liver development (e.g., glucose-6-phosphatase and α1-antitrypsin). However, genes expressed around the time of birth or in the immediate postnatal period, such as mdr-1b, serine dehydratase, and tyrosine aminotransferase, are not induced (16). In addition, certain liver-enriched transcription factors are either not induced (HNF-3α) or are induced only weakly (HNF-1α and HNF-4). This may at least in part account for the lack of a fully mature hepatocyte phenotype in this model (16).
Based on these observations, we hypothesized that the adult pancreas and liver retain common progenitor cells that upon activation can proliferate and differentiate along a specific foregut epithelial cell lineage (9, 16). To test this hypothesis and to determine the differentiation potential of putative pancreatic epithelial progenitor cells, we isolated and transplanted genetically marked cells into the liver of an inbred strain of mutant rats in which we could follow the fate of transplanted cells. Normal Fischer (F344) rats express a specific exopeptidase, dipeptidyl peptidase IV (DPPIV), in a characteristic pattern in the liver, restricted to the apical domain of the plasma membrane (17–19). This unique pattern of expression is similar to that observed with ATPase, a classical marker of the hepatocyte bile canaliculus (20). A mutant strain of F344 rats has been identified in which DPPIV enzyme activity is not expressed (21), and a monoclonal antibody, Mab 236.3, which recognizes the normal but not the mutant DPPIV protein, has also been raised (21). In this study, we simultaneously detected both DPPIV and ATPase by histochemical methods (22) to identify and characterize transplanted DPPIV+ pancreatic epithelial cells in the DPPIV− recipient liver and their relationship to endogenous hepatocytes.
MATERIALS AND METHODS
Animals and Diets.
Male Fischer rats (F344, a highly inbred strain) were purchased from Charles River Breeding Laboratories. DPPIV− mutant F344 rats, provided by D. Hixson (21), were bred and maintained in the Special Animal Core of the Liver Research Center, Albert Einstein College of Medicine. A Cu-deficient diet was purchased from United States Biochemicals. The copper chelator, triethylene tetramine (Trien), purchased from either Aldrich or Sigma, was used at a concentration of 0.6% (wt/vol). Animals were maintained on this diet for 8–10 weeks, as described by Rao et al. (14, 15). The initial weight of the rats was ≈80 g. Multiple animals were used for tissue analysis and isolation of pancreatic cells. All studies were conducted under protocols approved by the Animal Care Use Committee of the Albert Einstein College of Medicine and were in accordance with National Institutes of Health policy (23).
Pancreatic and Liver Epithelial Cell Isolation.
The pancreas was perfused through a retrograde aortic catheter with 300 ml Leffert’s EGTA solution (24) followed by perfusion with 80 ml of Dulbecco’s modified Eagle’s (DME)/F12 medium (GIBCO) containing 15 mM Hepes (pH 7.4), 1 mg/ml type IV collagenase (Sigma), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B (Sigma). The perfused pancreas was excised and cut into small pieces. Minced pancreas was incubated for 40 min at 37°C in DME/F12 medium containing 15 mM Hepes (pH 7.5), 1 mg/ml type IV collagenase, 0.1 mg/ml soybean trypsin inhibitor, 0.04 mg/ml DNase I (Boehringer Mannheim), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B. Digested tissue was mechanically disrupted through a glass Pasteur pipette and Falcon plastic pipette and subsequently passed through an 80-μM nylon mesh. Cells were collected by centrifugation for 5 min at 300 × g, washed two times with DME/F12 medium, suspended in 11% Nycodenz prepared in a buffer containing 0.24 g Hepes, 0.5 g KCl, 0.018 g CaCl2 H2O, and 5.5 ml 0.1 N NaOH in 100 ml (pH 7.6), and fractionated through a discontinuous Nycodenz gradient (11%, 13%, 19%, and 30% wt/vol) for 30 min at 8,000 rpm in a Beckman SW rotor at 4°C. Cells at the top of the gradient and at the interfaces between the various layers were collected and termed Fx-1 (<11% Nycodenz), Fx-2 (11–13% Nycodenz interface), Fx-3 (13–19% Nycodenz interface), and Fx-4 (19–30% Nycodenz interface), respectively. Cells in the various fractions were counted in a hemocytometer, pelleted at 300 × g for 5 min, and resuspended in 0.5 ml of DME/F12 medium.
Liver nonparenchymal epithelial cells were isolated from 180 g male F344 rats, 2.5 days after inducing acute hepatic necrosis by intraperitoneal injection of d-galactosamine (70 mg per 100 g body weight), using a modification of the procedure of Berry and Friend (25). The liver was cannulated through the portal vein, flushed with 250 ml Leffert’s solution containing EGTA (24), followed by 100 ml Leffert’s solution, and finally perfused with Leffert’s solution containing 3 mM CaCl2, and 0.1% type IV collagenase (Sigma) for 10–15 min at 37°C. The perfusate containing the bulk of hepatocytes was removed and the liver stromal remnant digested with Leffert’s solution, 3 mM CaCl2, 0.35% type IV collagenase (Sigma), 0.1% pronase (Boehringer Mannheim), and 0.005% DNase 1 (Boehringer Mannheim) for 30 min at 37°C to release nonparenchymal cells. The digested remnant was filtered through an 80-μM nylon mesh and suspended cells were enriched for nonparenchymal epithelial cells by three cycles of centrifugation at 50 × g for 1 min at 4°C (to remove residual hepatocytes), decantation of the supernatant fraction, sedimentation of cells in the supernatant fraction by centrifugation at 300 × g for 5 min at room temperature (RT) and resuspension of pelleted cells in DME/F12 medium (GIBCO), containing 15 mM Hepes (pH 7.4), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B. After this procedure, 30–40% of the cells in the resuspended fraction were positive for DPPIV.
Pancreatic and Hepatic Histology and in Situ Hybridization.
Tissue samples were fixed in formalin or snap frozen in 2-methylbutane at −70°C. Formalin-fixed tissue was embedded in paraffin, cut into 6-μm thick sections, and stained with hematoxylin and eosin or processed for in situ hybridization as described (16). Data presented in each figure are representative of data obtained from at least three separate experiments.
Detection of DPPIV and ATPase Enzyme Activity.
DPPIV enzyme expression was determined on cytospin slides stored at −20°C or on 5-μM thick cryostat sections from frozen tissue. Fixation was for 5 min in 95% ethanol/5% glacial acetic acid (99:1 vol/vol) at 0°C to −10°C, followed by a 5-min wash in 95% ethanol at 4°C. Air-dried slides were incubated for 10–20 min at 37°C in the substrate reagent: 2.5 mg Gly-Pro-4-methoxy-β-naphthylamide (Sigma) dissolved in 150 ml of dimethylformamide and mixed with a 5 ml solution of Fast blue BB salt (Sigma) in 0.1 M Tris maleate, 0.1 M NaCl, pH 6.5 (TMS). The slides were rinsed two times in TMS, incubated for 2 min in 0.1 M CuSO4, and rinsed again in TMS. The slides were fixed for 5–10 min in cold 4% paraformaldehyde in 0.15 M NaCl, washed two times in cold 0.2 M Tris maleate (pH 7.2), and processed when necessary for ATPase staining.
Histochemical staining for ATPase was carried out as described by Wachstein and Meisel (20). Slides were incubated for 1–2 hr at 37°C in the following substrate reagent: 25 mg ATP, sodium salt in 0.1 M Tris maleate buffer (pH 7.2), 0.01 M MgSO4, and 0.12% Pb(NO3)2, and rinsed in distilled water. Staining was developed by incubating the slides for 1–2 min at RT in dilute ammonium sulfide (0.05–0.2%). The slides were washed in water, counterstained with hematoxylin, and mounted in pure glycerol.
Immunohistochemistry and Confocal Laser Scanning Microscopy.
Cryostat sections (5 μM) were fixed in cold 4% paraformaldehyde in PBS buffer containing 5% sucrose for 10 min; treated with sodium borohydrate (1 mg/ml PBS) for 30 min at RT; blocked in a solution containing 2% goat serum, 1% BSA, and 0.1% Tween in PBS for 2 hr at RT; exposed to a mixture of mouse monoclonal antibody (ascites) to DPPIV (Mab 236.3) and rabbit polyclonal anti-rat albumin antibody (final dilution 1:1 and 1:100, respectively) for 2 hr or overnight in the cold; rinsed in PBS; and exposed to a mixture of goat anti-mouse IgG-Cy 3 and goat anti-rabbit IgG-Cy 5 (final dilution 1:100) for 2 hr or overnight in the cold. Primary antibodies were diluted in the blocking solution. After rinsing, sections were mounted with antifade mounting medium containing N-propyl gallate, PBS, glycerin, and examined with an inverted Nikon fluorescence microscope attached to the Bio-Rad MRC 600 confocal laser imaging system equipped with a krypton/argon laser and a Nikon 60K numerical aperture 1.4 Planapo objective.
For detection of cytokeratin (CK)-19, 5-μm cryosections were fixed for 15 min in cold acetone/ethanol (1:1) and endogenous peroxidase was blocked with H2O2. Further blocking was performed as described in the Vector ABC Elite kit (Vector Laboratories). CK-19 antibody (RPN 1165, Amersham) was applied for 2 hr at RT in a dilution of 1:10. Secondary antibody was from the ABC Elite kit and peroxidase was developed by diaminobenzidine staining. A negative control in which the primary antibody was omitted was included in all analyses.
RESULTS
Induction of Pancreatic Proliferation.
As shown in Fig. 1 A and B, after 8 weeks of feeding DPPIV+ F344 rats a Cu-deficient diet supplemented with 0.6% wt/vol Trien, there was a marked reduction of pancreatic acinar mass and remaining acinar cells showed a loss of much of their cytoplasmic contents (degranulation). Epithelial cells comprising the pancreatic ducts were detected by CK-19 expression (Fig. 1 C and D) and DPPIV enzyme activity (Fig. 1 G and H). In normal pancreas, both large ducts and small ductules between the acini stained with antibody to CK-19 (Fig. 1C) and DPPIV (Fig. 1G). After 8 weeks of Cu deficiency, the number of CK-19 or DPPIV positive cells in duct-like clusters and the size of these clusters increased substantially (Fig. 1 D and H, respectively). Pancreatic islet cells were not affected by Cu depletion.
Figure 1.
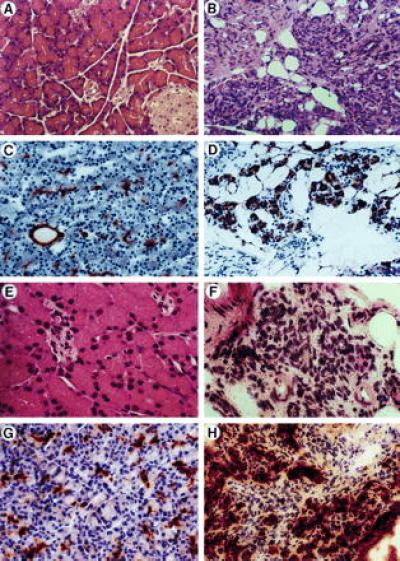
Characterization of epithelial cells in the pancreas after feeding F344 rats a Cu-deficient diet. Formalin fixed or snap frozen pancreatic tissues were collected from paired rats either untreated (A, C, E, and G) or treated (B, D, F, and H) with a Cu-deficient diet for 8 weeks. (A and B) Stained with hematoxylin/eosin. (C and D) Stained with CK-19 antibody. (E and F) Analyzed for albumin mRNA by in situ hybridization. (G and H) Analyzed for DPPIV by enzyme histochemistry. (A–D, G, and H, ×200; E and F, ×400).
Albumin expression, detected by in situ hybridization for albumin mRNA, was negative in the normal pancreas (Fig. 1E) but was present at high levels in the Cu-deficient pancreas (Fig. 1F). Albumin mRNA positive cells were distributed in the ductular and periductular spaces and were seen as individual cells or in small clusters forming duct-like structures. However, at no time during this study did we observe cells in the normal or Cu-deficient pancreas with the morphologic appearance of hepatocytes and also expressing albumin mRNA or DPPIV enzyme activity.
Isolation of Cell Fraction from Rat Pancreas Expressing Albumin mRNA.
Cells were isolated from the Cu-deficient F344 rat pancreas and fractionated on a four layer, discontinuous Nycodenz gradient. Cells banding at the top of the gradient and at the interfaces between the layers were separated into fractions (Fx) 1–4 and cytospun onto microscopic slides for histochemical analysis or in situ hybridization. Expression of DPPIV enzyme activity in the various cell fractions was used to determine the distribution of epithelial cells. As shown in Fig. 2 (A–D), DPPIV was expressed in small cells that were most abundant in Fx-3 (Fig. 2C), with spillover into Fx-4 (Fig. 2D). Occasionally, weak expression of DPPIV was also noted in some cells in Fx-1 (Fig. 2A). The origin of these cells is unclear, although they are slightly larger than DPPIV positive cells in Fx-3 and are present in normal pancreas.
Figure 2.
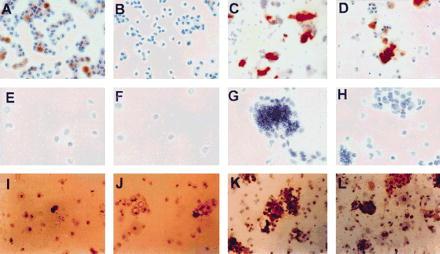
Expression of an epithelial cell marker (DPPIV), a liver-specific gene (albumin), and a pancreatic/biliary ductal cell marker (CK-19) in cell fractions isolated from the Cu-deficient rat pancreas. Cell fractions 1–4 collected at different densities in a discontinuous Nycodenz gradient were obtained as noted in Materials and Methods. Fractions 1–4 are displayed from left to right. A–D are stained histochemically for DPPIV (×100), E–H are hybridized for albumin mRNA (×200) using a 35S-labeled antisense albumin RNA riboprobe as previously reported (7), and I–L are immunohistochemically stained for CK-19 (×100) using a monoclonal anti-CK-19 as primary antibody (Amersham) and polyvalent anti-mouse immunoglobulin peroxidase conjugate (Sigma) as secondary antibody.
To identify pancreatic epithelial cells that have entered the hepatocyte lineage program, we performed in situ hybridization for albumin mRNA (Fig. 2 E–H). A strong hybridization signal was observed in cells distributed primarily in Fx-3 (Fig. 2G), although once again there was spillover into Fx-4 (Fig. 2H). These cells were of very small size (≈8–10 μM), with an oval shaped nucleus and scant cytoplasm, consistent with the DPPIV+ epithelial cells. No hepatocytes were observed in these cell fractions. Cells expressing either DPPIV or albumin tended to clump on centrifugation and appeared as aggregates on cytospin slides. Histochemical analysis for CK-19 (Fig. 2 I–L) also showed a distribution predominantly in Fx-3 (Fig. 2K). For each of these markers, up to 30–40% of cells in Fx-3 were positive, and this fraction contained 80–90% of total cells expressing DPPIV, albumin mRNA, and CK-19.
Transplantation of Pancreatic and Liver Epithelial Progenitor Cells.
Pancreatic cells were delivered to the liver by two established routes: one via transplantation to the spleen and the other via infusion into the portal vein. Approximately 0.3–2 million pancreatic cells from the individual fractions were transplanted into each recipient. Animals were killed 6 weeks to 3 months after cell transplantation. The spleen and liver (or the liver only when the portal vein was used for cell transplantation) were excised, snap frozen in 2-methylbutane at −70°C, and serial sections prepared. Every third section was stained for DPPIV enzyme activity. Using dual DPPIV/ATPase staining, we also assessed the structural relationship of DPPIV-positive cells to endogenous hepatocytes. DPPIV-positive cells remaining in the spleen after transplantation of cell Fx-3 were observed in clusters and retained an undifferentiated epithelial morphology or formed small or large duct structures (data not shown). DPPIV-positive cells with an hepatocyte-like morphology were not observed in the spleen. After transplantation of epithelial cells of Fx-3 from the Cu-deficient rat pancreas into the liver, large DPPIV-positive cells with a distinct hepatocyte-like morphology and the physical dimensions of hepatocytes (≈30–40 μM diameter) were found singly or in clusters, ranging in size up to 20 or more cells per cluster on cross section (Fig. 3A). DPPIV expression in these cells was unique and characteristic of the hepatocyte bile canaliculus (Fig. 3A). DPPIV staining in transplanted cells was contiguous with ATPase staining in adjacent endogenous hepatocytes, forming hybrid canaliculi similar to those reported recently following hepatocyte transplantation (22).
Figure 3.
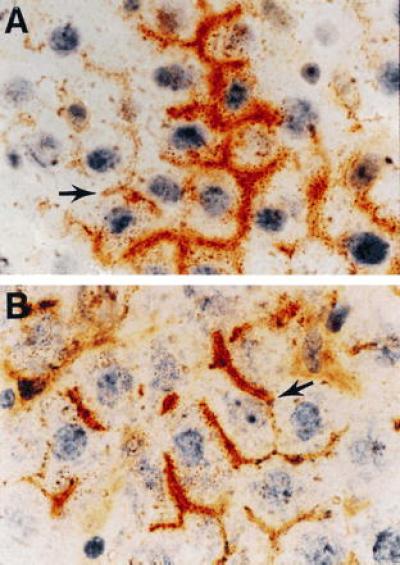
Identification of DPPIV-positive pancreatic and liver epithelial cells transplanted to the liver of DPPIV-negative mutant F344 rats. Dual histochemical staining of the tissue for DPPIV (orange/rust) and ATPase (brown) was conducted as noted. (A) Section of liver from a DPPIV-negative mutant F344 rat transplanted with an epithelial cell-enriched fraction from the pancreas of an 8-week-treated Cu-deficient DPPIV-positive F344 rat (×600). (B) Section of liver from a DPPIV-negative mutant rat transplanted with an epithelial cell-enriched fraction from the liver of a DPPIV-positive F344 rat, 2.5 days after treatment with d-galactosamine (×600). Arrows point to hybrid bile canaliculi formed between transplanted cells and endogenous hepatocytes.
Previously, we and Fausto’s group reported that d-galactosamine-induced liver injury activates proliferation and differentiation of hepatocyte progenitor or facultative stem cells (8, 9). To directly compare the morphologic appearance of transplanted pancreatic epithelial cells with epithelial progenitor cells derived from rat liver, we isolated a nonparenchymal cell fraction from the liver of a d-galactosamine-treated DPPIV+ F344 rat and transplanted this cell fraction into DPPIV− F344 rats. As shown in Fig. 3, the morphologic appearance, DPPIV staining pattern, and distribution of transplanted epithelial cells within the recipient parenchyma was indistinguishable with epithelial cells from either d-galactosamine-treated rat liver (Fig. 3B) or Cu-deficient rat pancreas (Fig. 3A). In both cases, transplanted cells were physically integrated into the parenchymal structure, forming hybrid canaliculi with adjacent endogenous hepatocytes (Fig. 3, arrows). Control transplantation experiments with the other epithelial cell fractions, as well as epithelial cell fractions from normal pancreas, were negative.
Differentiated Function and Hepatocytic Phenotype of Transplanted Cells.
To further demonstrate the hepatocytic phenotype of pancreatic epithelial progenitor cells transplanted into the liver, we used double-label immunofluorescent laser scanning confocal microscopy to detect albumin and DPPIV in the same cells. In the normal liver, hepatocytes expressed DPPIV in a canalicular distribution and albumin in a cytosolic distribution (Fig. 4A). In the DPPIV-mutant F344 rat liver, immunofluorescence for DPPIV was not observed, whereas albumin immunofluorescence was normal (Fig. 4B). In DPPIV-mutant F344 rats transplanted with DPPIV-positive pancreatic epithelial cells from Cu-deficient rats, clusters of transplanted cells with the morphologic appearance of hepatocytes were positive for both DPPIV in a canalicular distribution and albumin in a cytosolic distribution (Fig. 4C). By immunohistochemical analysis, albumin expression in transplanted cells was comparable to that observed in surrounding endogenous hepatocytes (Fig. 4C), suggesting full hepatic function of transplanted cells.
Figure 4.
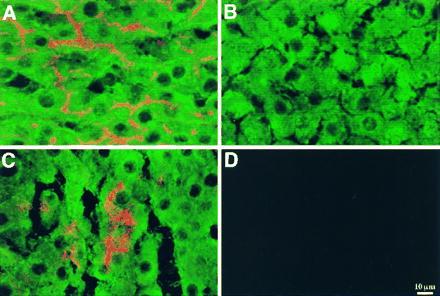
Colocalization of DPPIV and albumin expression in pancreatic epithelial cells from a Cu-deficient DPPIV-positive F344 rat transplanted to the liver of a DPPIV-negative mutant F344 rat. DPPIV and albumin were detected in the recipient liver by dual-label confocal laser scanning immunofluorescent microscopy. In normal rat liver (A), DPPIV expression (red/orange) is evident in bile canaliculi and albumin expression (green) in the cytoplasm of all the hepatocytes within the hepatic lobule. In DPPIV-negative mutant rat liver (B), no DPPIV is evident in any cell; however, all hepatocytes express albumin. In DPPIV-negative mutant rat liver transplanted with pancreatic epithelial cells from an 8-week-old Cu-deficient rat (C), DPPIV expression is evident in a cluster of hepatocytes, which are also expressing albumin at the same level as observed in surrounding endogenous hepatocytes, which are negative for DPPIV. In control normal liver (D), in the absence of primary antibodies, no fluorescent signal is detected. (Scale bar = 10 μM.)
DISCUSSION
Previously, we reported that Cu depletion in rats leads to proliferation of epithelial cells in the pancreas that express liver-specific genes at early and mid stages of hepatic lineage progression (16). The findings in this study that transplantation of these cells to the liver leads to their differentiation into mature hepatocytes with structural integration in the hepatic parenchyma and expression of biochemical functions unique to the hepatocyte, provides compelling evidence that these cells are indeed hepatocyte progenitors. To our knowledge, this represents the first example in which epithelial progenitor cells, activated to proliferate in one adult organ, were transplanted to a second adult organ and assumed the differentiated cell phenotype of the recipient organ. This finding is not unreasonable, since the pancreas and liver have a common embryologic origin (1–4). Our conclusion from this study and our previous findings (9, 16) is that residual multipotent cells of epithelial origin are present through adulthood in both the liver and pancreas. Under appropriate circumstances, these cells can be induced to proliferate and differentiate in a lineage-specific fashion, depending on their organ location and pathophysiologic circumstances.
After transplantation into the liver, there was no evidence of transformed behavior in pancreatic epithelial cells proliferating and differentiating along the hepatocyte lineage. Recently, studies by Coleman et al. (26) reported that certain established transformed epithelial cell lines derived from rat liver lose their malignant phenotype after transplantation into the liver and assume a normal hepatocyte morphology. Our study shows, additionally, that transplanted progenitor cells can fully integrate into the hepatic lobular structure, implying that they are functionally identical with endogenous hepatocytes. This is not surprising, since it has been shown previously that transplanted hepatocytes have the capacity to traverse the hepatic sinusoids and become incorporated into the hepatic parenchyma (22).
Chen et al. (27) recently reported that an epithelial cell line derived from normal adult rat pancreas has the ability to exhibit a differentiated, hepatocyte-like morphology after transplantation into the peritoneal cavity. These experiments required the cells to be embedded in a mixture of collagen type 1 and Matrigel, a basement membrane matrix material derived from Englebreth-Holm–Swarm tumor cells. When this cell line was transplanted subcutaneously under the same experimental conditions, a mixed biliary/hepatocytic/pancreatic epithelial cell phenotype was observed (27). These studies, those of Reddy and coworkers (14, 15), and our present findings imply that the specific tissue environment, extracellular conditions, and cell–cell contacts all contribute to the differentiation state of gut epithelial cells.
In previous studies using normal recipient hosts, hepatocytes or transformed liver epithelial cell lines undergo little, if any, proliferation after transplantation into the liver (22, 26). In contrast, in transgenic mice containing the urokinase plasminogen activator gene under control of the albumin promoter, and in whom there is a continuous state of liver injury/regeneration, transplanted hepatocytes undergo multiple rounds of division (28). A similar phenomenon occurs in furamylacetoacetate hydrolase knockout mice after transplantation of normal hepatocytes (29). However, for cell transplantation to become an effective means of liver reconstitution, similar results will need to be obtained in nongenetically manipulated animals (and humans).
Since transplanted epithelial progenitor cells differentiated into mature hepatocytes in this study, this suggests that cell lines derived from progenitor cells obtained from adult tissues may be useful for restoration of hepatic mass. These findings may have even broader implications for cell transplantation, as evidence for progenitor cells that are capable of being activated to proliferate and differentiate has also been obtained recently in other adult tissues, such as the brain (30), bone mesenchyme (31), and bronchial epithelium (32).
Acknowledgments
We thank Drs. C. and L. Rogler, R. Burk, J. Roy Chowdhury, and J. Kessler for reviewing this manuscript; M. Cammer of the Imaging and Cell Structure Core, Marion Bessin Liver Research Center, for assistance with confocal microscopy; E. Bobe and A. Caponigro for preparing the text; and J. Kwon for preparing the figures. This work was supported in part by National Institutes of Health Grants DK17609, DK50636, and P30 DK41296 to D.A.S., CA06576 to P.M.N., CA42715 to D.C.H., and DK46952 to S.G. S.-G.H. was supported by a fellowship from the College of Medicine, Soonhunkyang University (Seoul, Korea).
ABBREVIATIONS
- E
embryonic day
- DPPIV
dipeptidyl peptidase IV
- RT
room temperature
- CK
cytokeratin
References
- 1.DuBois A M. In: The Liver. Rouiller H, editor. New York: Academic; 1963. [Google Scholar]
- 2.Wilson J W, Groat C S, Leduc E. Ann NY Acad Sci. 1963;11:8–22. doi: 10.1111/j.1749-6632.1963.tb36945.x. [DOI] [PubMed] [Google Scholar]
- 3.Houssaint E. Cell Differ. 1980;9:269–279. doi: 10.1016/0045-6039(80)90026-3. [DOI] [PubMed] [Google Scholar]
- 4.Githens S. In: The Exocrine Pancreas: Biology, Pathobiology and Diseases. Go V L W, editor. New York: Raven; 1986. pp. 21–32. [Google Scholar]
- 5.Wilson J W, Leduc E H. J Pathol Bacteriol. 1958;76:441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- 6.Evarts R P, Nagy P, Marsden E, Thorgeirsson S S. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 7.Evarts R P, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson S S. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 8.Lemire J M, Shiojiri N, Fausto N. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- 9.Dabeva M D, Shafritz D A. Am J Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- 10.Grisham J W. Ann NY Acad Sci. 1980;349:128–136. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- 11.Sell S. Cancer Res. 1990;50:3811–3815. [PubMed] [Google Scholar]
- 12.Fausto N. Curr Opin Cell Biol. 1990;2:1036–1041. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- 13.Thorgeirsson S S. Am J Pathol. 1993;142:1331–1333. [PMC free article] [PubMed] [Google Scholar]
- 14.Rao M S, Dwivedi R S, Subbarao V, Usman M I, Scarpelli D G, Nemali M R, Yeldandi A, Thangada S, Kumar S, Reddy J K. Biochem Biophys Res Commun. 1988;156:131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 15.Rao M S, Dwivedi R S, Yeldandi A V, Subbarao V, Tan X, Usman M I, Thangada S, Nemali M R, Kumar S, Scarpelli D G. Am J Pathol. 1989;134:1069–1086. [PMC free article] [PubMed] [Google Scholar]
- 16.Dabeva M D, Hurston E, Shafritz D A. Am J Pathol. 1995;147:1633–1648. [PMC free article] [PubMed] [Google Scholar]
- 17.Gossrau R. Histochemistry. 1979;60:231–248. doi: 10.1007/BF00495756. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard A L, Bartles J R, Braiterman L T. J Cell Biol. 1985;100:1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walborg E F, Jr, Tsuchida S, Weeden D S, Thomas M W, Barrick A, McEntire K D, Allison J P, Hixson D C. Exp Cell Res. 1985;158:509–518. doi: 10.1016/0014-4827(85)90474-4. [DOI] [PubMed] [Google Scholar]
- 20.Wachstein M, Meisel E. Am J Clin Pathol. 1955;27:13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Thompson N L, Hixson D C, Callavan H, Panzica M, Flanagan D, Faris R A, Hong W, Hartel-Schenk S, Doyle D. Biochem J. 1991;273:497–502. doi: 10.1042/bj2730497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Rajvanshi P, Lee C-D. Proc Natl Acad Sci USA. 1995;92:5860–5864. doi: 10.1073/pnas.92.13.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl. Inst. Health; 1985. , DHHS Publ. No. (NIH) 86–23. [Google Scholar]
- 24.Leffert H L, Koch K S, Moran T, Williams M. Methods Enzymol. 1979;58:536–544. doi: 10.1016/s0076-6879(79)58168-3. [DOI] [PubMed] [Google Scholar]
- 25.Berry M N, Friend D S. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman W B, Wennerberg A E, Smith G J, Grisham J W. Am J Pathol. 1993;142:1373–1382. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J-R, Tsao M-S, Duguid W P. Am J Pathol. 1995;147:707–717. [PMC free article] [PubMed] [Google Scholar]
- 28.Rhim J, Sangren E P, Degen J L, Palmiter R D, Brinster R L. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 29.Overturf K, Muhsen A-D, Tanguay R, Brantly M, Ou C-N, Finegold M, Grompe M. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds B A, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 31.Caplan A I. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 32.Zepeda M L, Chinoy M R, Wilson J M. Somatic Cell Mol Genet. 1995;21:61–73. doi: 10.1007/BF02255823. [DOI] [PubMed] [Google Scholar]


