Abstract
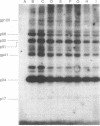
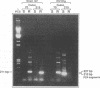
Drug-resistant variants of human immunodeficiency virus type 1 (HIV-1) have been isolated by in vitro selection. MT-4 cells were infected with either a laboratory strain (HIV-IIIB) or a clinical isolate (no. 187) of HIV-1 and maintained in medium containing subeffective concentrations of the drugs 3'-azido-3'-deoxythymidine (AZT) and 2',3'-dideoxyinosine (ddI). By gradually increasing the drug concentration in the culture medium during propagation of the virus on fresh MT-4 cells, we were able to isolate variants of HIV-IIIB and clinical isolate 187 which showed up to 100-fold increases in resistance to the drugs. The drug resistance phenotypes remained stable after propagation of the variants in the absence of drug pressure for over 2 months. However, variants resistant to one drug showed little or no cross-resistance to the other, suggesting that the genetic bases for resistance to the compounds differed. Genotypic analysis of these nucleoside-resistant variants by polymerase chain reaction (PCR) with primer pairs previously shown to correspond to mutations responsible for resistance to AZT was also carried out. A heterogeneity of genotypes was observed, with known mutations at pol codons 70 and 215 occurring in most of the AZT-resistant variants generated from either HIV-IIIB or clinical strain 187. However, mutations in codons 67 and 219 were less frequently detected, and none of these changes were observed in each of four variants resistant to ddI. Cloning and sequencing studies of the reverse transcriptase coding region of two of the isolates were also performed and confirmed the PCR data that had been obtained. In addition to previously described mutation sites responsible for resistance to AZT, an HIV-IIIB-resistant variant was shown to be mutated at positions 108 (Val----Ala) and 135 (Ile----Thr), while a resistant variant of strain 187 was mutated at positions 50 (Ile----Val) and 135 (Ile----Val).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biron K. K., Fyfe J. A., Stanat S. C., Leslie L. K., Sorrell J. B., Lambe C. U., Coen D. M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. The activity in vitro against herpes virus of 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine), a new antiviral agent. J Antimicrob Chemother. 1979 Jul;5(4):431–436. doi: 10.1093/jac/5.4.431. [DOI] [PubMed] [Google Scholar]
- Collins P., Larder B. A., Oliver N. M., Kemp S., Smith I. W., Darby G. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989 Feb;70(Pt 2):375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., 2nd Molecular targets of antiviral therapy. N Engl J Med. 1989 Jul 20;321(3):163–172. doi: 10.1056/NEJM198907203210306. [DOI] [PubMed] [Google Scholar]
- Desai S. M., Kalyanaraman V. S., Casey J. M., Srinivasan A., Andersen P. R., Devare S. G. Molecular cloning and primary nucleotide sequence analysis of a distinct human immunodeficiency virus isolate reveal significant divergence in its genomic sequences. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8380–8384. doi: 10.1073/pnas.83.21.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K. K., Stanat S. C., Balfour H. H., Jr, Jordan M. C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989 Feb 2;320(5):289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Gill P. S., Rarick M., Brynes R. K., Causey D., Loureiro C., Levine A. M. Azidothymidine associated with bone marrow failure in the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1987 Oct;107(4):502–505. doi: 10.7326/0003-4819-107-4-502. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of human T-lymphotropic virus type-I (HTLV-I)-bearing MT-4 cells with HTLV-III (AIDS virus): chronological studies of early events. Virology. 1985 Oct 30;146(2):272–281. doi: 10.1016/0042-6822(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Wolstenholme A. J., Skehel J. J., Smith M. H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985 Nov;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japour A. J., Chatis P. A., Eigenrauch H. A., Crumpacker C. S. Detection of human immunodeficiency virus type 1 clinical isolates with reduced sensitivity to zidovudine and dideoxyinosine by RNA.RNA hybridization. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3092–3096. doi: 10.1073/pnas.88.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W., Kaufman E. R., Crumpacker C. Physical mapping of drug resistance mutations defines an active center of the herpes simplex virus DNA polymerase enzyme. J Virol. 1981 Sep;39(3):746–757. doi: 10.1128/jvi.39.3.746-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Coates K. E., Kemp S. D. Zidovudine-resistant human immunodeficiency virus selected by passage in cell culture. J Virol. 1991 Oct;65(10):5232–5236. doi: 10.1128/jvi.65.10.5232-5236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kellam P., Kemp S. D. Zidovudine resistance predicted by direct detection of mutations in DNA from HIV-infected lymphocytes. AIDS. 1991 Feb;5(2):137–144. doi: 10.1097/00002030-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck M. D., Schulman J. L., Palese P. Susceptibility of influenza A viruses to amantadine is influenced by the gene coding for M protein. J Virol. 1978 Dec;28(3):710–716. doi: 10.1128/jvi.28.3.710-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. W., North G. L., Pedersen N. C. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1989 Jun;33(6):915–919. doi: 10.1128/aac.33.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Schleif W. A., Boots E. J., O'Brien J. A., Quintero J. C., Hoffman J. M., Emini E. A., Goldman M. E. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J Virol. 1991 Sep;65(9):4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. D., Poiesz B. J., Loeb L. A. Fidelity of HIV-1 reverse transcriptase. Science. 1988 Nov 25;242(4882):1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- Remington K. M., Chesebro B., Wehrly K., Pedersen N. C., North T. W. Mutants of feline immunodeficiency virus resistant to 3'-azido-3'-deoxythymidine. J Virol. 1991 Jan;65(1):308–312. doi: 10.1128/jvi.65.1.308-312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Bebenek K., Kunkel T. A. The accuracy of reverse transcriptase from HIV-1. Science. 1988 Nov 25;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- Rooke R., Tremblay M., Soudeyns H., DeStephano L., Yao X. J., Fanning M., Montaner J. S., O'Shaughnessy M., Gelmon K., Tsoukas C. Isolation of drug-resistant variants of HIV-1 from patients on long-term zidovudine therapy. Canadian Zidovudine Multi-Centre Study Group. AIDS. 1989 Jul;3(7):411–415. doi: 10.1097/00002030-198907000-00001. [DOI] [PubMed] [Google Scholar]
- Rooke R., Tremblay M., Wainberg M. A. AZT (zidovudine) may act postintegrationally to inhibit generation of HIV-1 progeny virus in chronically infected cells. Virology. 1990 May;176(1):205–215. doi: 10.1016/0042-6822(90)90245-m. [DOI] [PubMed] [Google Scholar]
- Ruprecht R. M., Chou T. C., Chipty F., Sosa M. G., Mullaney S., O'Brien L., Rosas D. Interferon-alpha and 3'-azido-3'-deoxythymidine are highly synergistic in mice and prevent viremia after acute retrovirus exposure. J Acquir Immune Defic Syndr. 1990;3(6):591–600. [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Skolnik P. R., Kosloff B. R., Hirsch M. S. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988 Mar;157(3):508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- Smith M. S., Brian E. L., Pagano J. S. Resumption of virus production after human immunodeficiency virus infection of T lymphocytes in the presence of azidothymidine. J Virol. 1987 Dec;61(12):3769–3773. doi: 10.1128/jvi.61.12.3769-3773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Nagumo T., Hoshino H. Low fidelity of cell-free DNA synthesis by reverse transcriptase of human immunodeficiency virus. J Virol. 1988 Oct;62(10):3900–3902. doi: 10.1128/jvi.62.10.3900-3902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M., Sullivan A. K., Rooke R., Geleziunas R., Tsoukas C., Shematek G., Gilmore N., Wainberg M. A. New CD4(+) cell line susceptible to infection by HIV-1. J Med Virol. 1989 Aug;28(4):243–249. doi: 10.1002/jmv.1890280408. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Broder S. Development of antiretroviral therapy for the acquired immunodeficiency syndrome and related disorders. A progress report. N Engl J Med. 1987 Feb 26;316(9):557–564. doi: 10.1056/NEJM198702263160925. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Klecker R. W., Weinhold K. J., Markham P. D., Lyerly H. K., Durack D. T., Gelmann E., Lehrman S. N., Blum R. M., Barry D. W. Administration of 3'-azido-3'-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986 Mar 15;1(8481):575–580. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science. 1986 Mar 14;231(4743):1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]