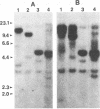
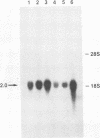
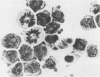
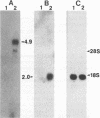
Abstract
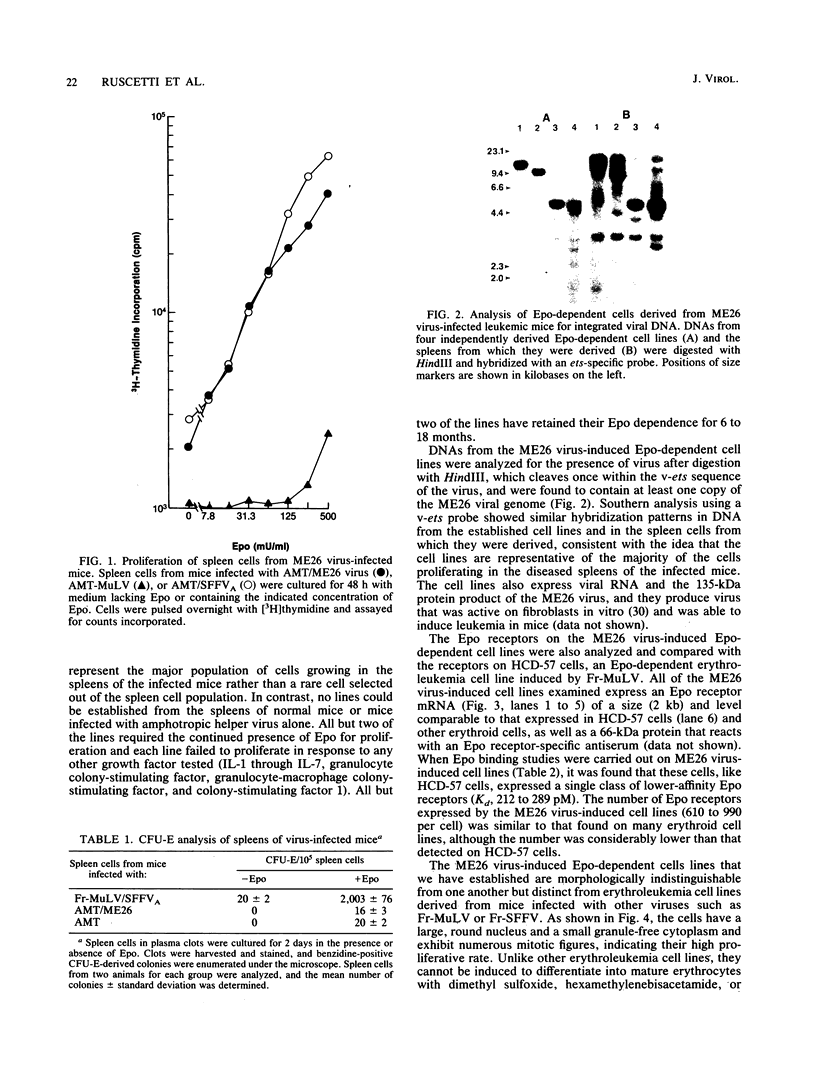
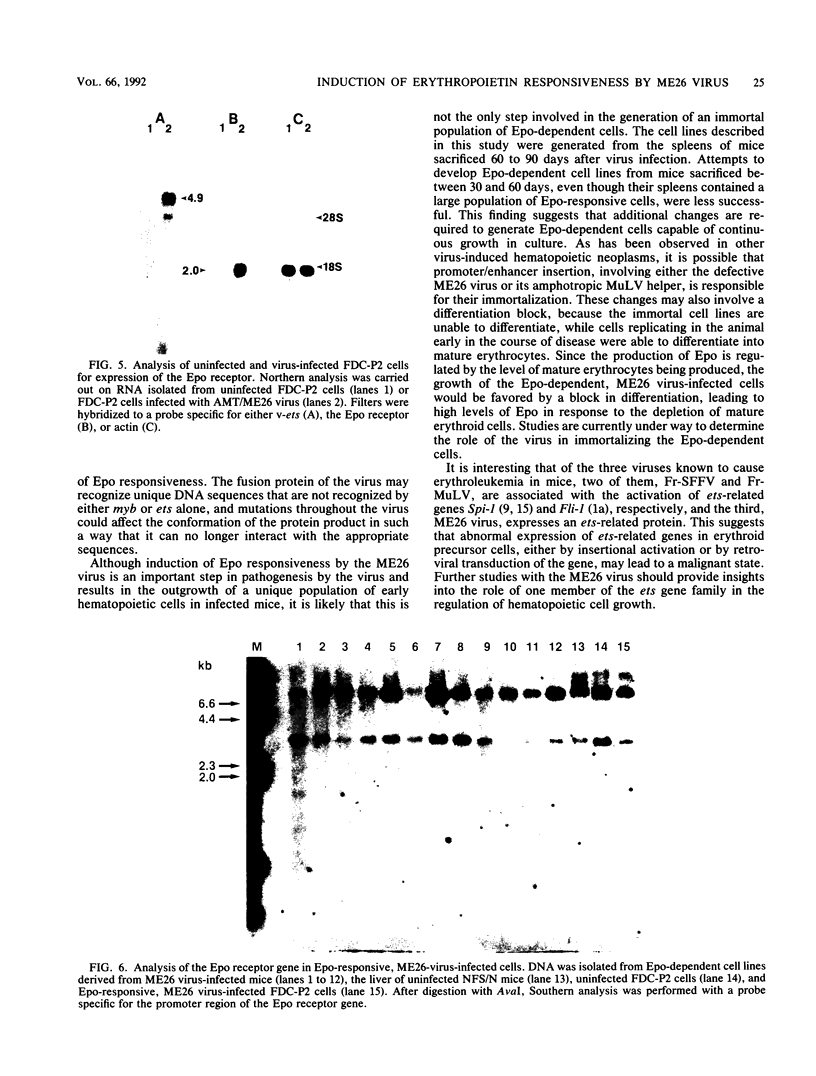
ME26 virus, which was generated by inserting the coding region of the acute avian leukemia-inducing virus E26 into a murine retrovirus vector, encodes a 135-kDa gag-myb-ets fusion protein. Amphotropic murine leukemia virus pseudotypes of ME26 virus induce a high incidence of erythroleukemia 2 to 4 months after injection into newborn NFS/N mice. Spleen cells from the majority of these mice proliferate to high levels in the presence of the erythroid hormone erythropoietin (Epo) and can easily be established as permanent Epo-dependent cell lines. The cell lines contain multiple copies of ME26 viral DNA and express viral message and protein. An Epo receptor mRNA of normal size can be detected in these cells, and binding studies reveal a single class of lower-affinity Epo receptor with an affinity for Epo that is in the range of that previously reported for erythroid cells. The ME26 virus-induced Epo-dependent cell lines, however, appear more immature than previously described erythroid cell lines and more closely resemble early hematopoietic precursor cells, suggesting that the virus may be activating the Epo receptor in hematopoietic cells that do not normally express it. Consistent with this idea, we are able to infect an interleukin-3-dependent myeloid cell line, FDC-P2, with ME26 virus and convert it to Epo dependence. The ME26 virus-infected FDC-P2 cells, even before growth on Epo, showed a large increase in the amount of Epo receptor mRNA. However, no ME26 viral integrations can be detected adjacent to the Epo receptor gene, indicating that the virus is not activating the Epo receptor gene by promoter/enhancer insertion. Our results are more consistent with the hypothesis that the gag-myb-ets-encoded viral fusion protein, which is known to bind DNA, is directly or indirectly activating the expression of the Epo receptor gene in these cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. trans-Activation of a globin promoter in nonerythroid cells. Mol Cell Biol. 1991 Feb;11(2):843–853. doi: 10.1128/mcb.11.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M. K. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell. 1990 Jun 29;61(7):1165–1166. doi: 10.1016/0092-8674(90)90676-6. [DOI] [PubMed] [Google Scholar]
- Hara H., Ogawa M. Erthropoietic precursors in mice with phenylhydrazine-induced anemia. Am J Hematol. 1976;1(4):453–458. doi: 10.1002/ajh.2830010410. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Metz T., Graf T. v-myb and v-ets transform chicken erythroid cells and cooperate both in trans and in cis to induce distinct differentiation phenotypes. Genes Dev. 1991 Mar;5(3):369–380. doi: 10.1101/gad.5.3.369. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Ray D., Mattei M. G., Tambourin P., Tavitian A. The putative oncogene Spi-1: murine chromosomal localization and transcriptional activation in murine acute erythroleukemias. Oncogene. 1989 Dec;4(12):1449–1456. [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K. Employment of a [3H]thymidine-incorporation assay to distinguish the effects of different Friend erythroleukemia-inducing retroviruses on erythroid cell proliferation. J Natl Cancer Inst. 1986 Jul;77(1):241–245. [PubMed] [Google Scholar]
- Ruscetti S. K., Janesch N. J., Chakraborti A., Sawyer S. T., Hankins W. D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990 Mar;64(3):1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Biological and biochemical differences between variants of spleen focus-forming virus can be localized to a region containing the 3' end of the envelope gene. J Virol. 1985 Dec;56(3):717–722. doi: 10.1128/jvi.56.3.717-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Goldwasser E. Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem. 1987 Apr 25;262(12):5554–5562. [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Papas T. S. Molecular organization of the chicken ets locus. Virology. 1988 May;164(1):99–105. doi: 10.1016/0042-6822(88)90624-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Youssoufian H., Zon L. I., Orkin S. H., D'Andrea A. D., Lodish H. F. Structure and transcription of the mouse erythropoietin receptor gene. Mol Cell Biol. 1990 Jul;10(7):3675–3682. doi: 10.1128/mcb.10.7.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C. C., Kan N., Dunn K. J., Papas T. S., Blair D. G. Properties of a murine retroviral recombinant of avian acute leukemia virus E26: a murine fibroblast assay for v-ets function. J Virol. 1989 Jan;63(1):205–215. doi: 10.1128/jvi.63.1.205-215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]