Abstract
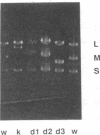
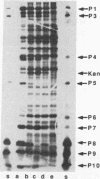
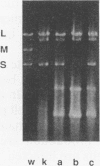
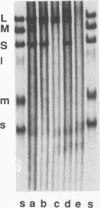
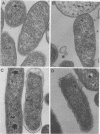
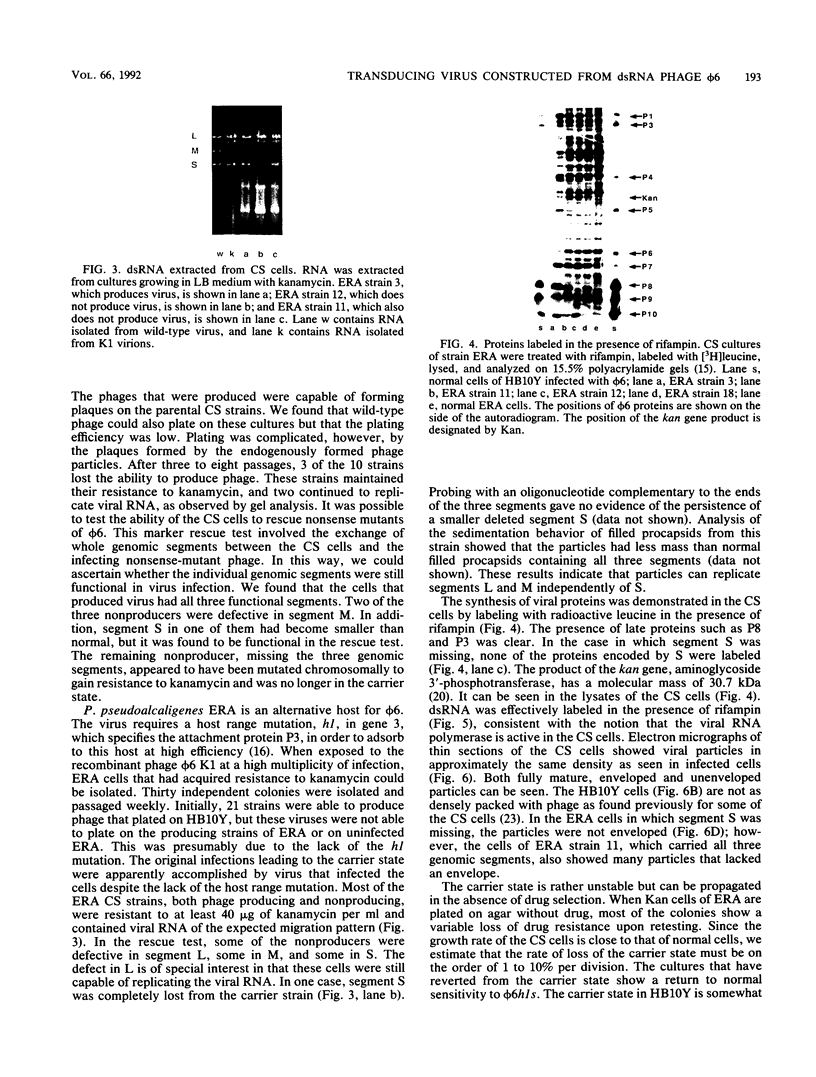
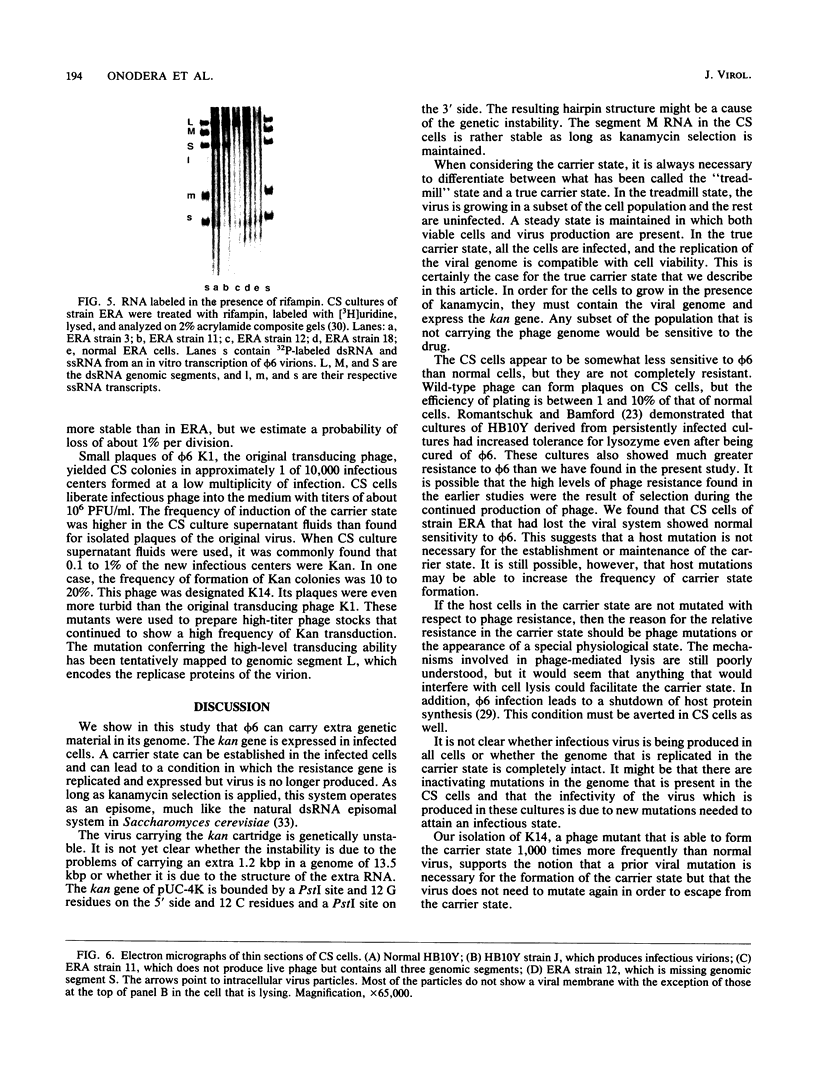
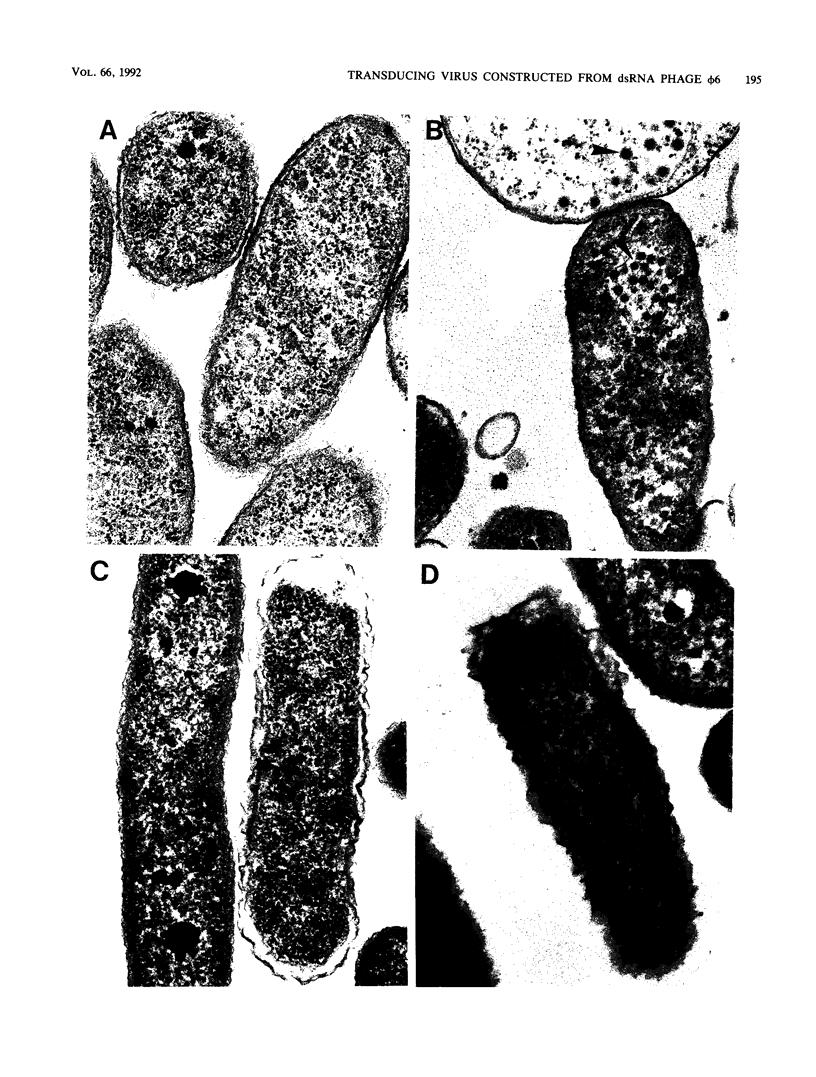
Bacteriophage phi 6 contains three double-stranded RNA (dsRNA) genomic segments. We have constructed a plasmid that contains a cDNA copy of the middle (M) segment, with a gene for kanamycin resistance (kan) inserted into the PstI site. A transcript of this cDNA was incorporated in vitro into procapsids along with natural transcripts of the S and L segments. The procapsids were coated with nucleocapsid surface protein P8 and transfected into Pseudomonas syringae pv. phaseolicola. The resulting infectious virus, phi 6 K1, was found to contain an M segment that was 1.2 kbp larger than the normal 4.1 kbp. K1 formed small, turbid plaques, and its genome was unstable. Preparations of K1 contained from about 0.1 to 10% large, clear-plaque forms of the virus which were usually missing the kan gene, and in some cases, the resulting segment M was smaller than its normal size. Cells picked from lawns of host cells infected with K1 yielded colonies that were resistant to kanamycin (Kan). These colonies could be passaged on kanamycin-containing medium. The cells were found to contain large amounts of dsRNA corresponding to the viral genomic segments. Some strains continued to produce viable phage, while others lost this ability. One strain completely lost the small genomic segment S. Approximately 1 in 10,000 infected cells acquired the carrier state with the original phage isolate K1. However, we isolated a viral mutant that was able to induce the carrier state in 10 to 20% of the infected cells. The ability to use drug resistance as a test for the carrier state makes this system very useful for the study of the mechanisms of induction of persistent infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Ahmed R., Fields B. N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982 Mar;28(3):605–612. doi: 10.1016/0092-8674(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Mindich L. Electron microscopy of cells infected with nonsense mutants of bacteriophage phi 6. Virology. 1980 Nov;107(1):222–228. doi: 10.1016/0042-6822(80)90287-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Transcriptional regulation of three double-stranded RNA segments of bacteriophage phi 6 in vitro. J Virol. 1983 Apr;46(1):196–203. doi: 10.1128/jvi.46.1.196-203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Bamford D. H., Mindich L. Production of a polyhedral particle in Escherichia coli from a cDNA copy of the large genomic segment of bacteriophage phi 6. J Virol. 1988 Jan;62(1):181–187. doi: 10.1128/jvi.62.1.181-187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X. Y., Frucht A., Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990 Oct;172(10):5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. F., Mindich L. The isolation of new mutants of bacteriophage phi 6. Virology. 1979 Aug;97(1):164–170. doi: 10.1016/0042-6822(79)90382-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mindich L. Bacteriophage phi 6: a unique virus having a lipid-containing membrane and a genome composed of three dsRNA segments. Adv Virus Res. 1988;35:137–176. doi: 10.1016/s0065-3527(08)60710-1. [DOI] [PubMed] [Google Scholar]
- Mindich L., Bamford D., Goldthwaite C., Laverty M., Mackenzie G. Isolation of nonsense mutants of lipid-containing bacteriophage PRD1. J Virol. 1982 Dec;44(3):1013–1020. doi: 10.1128/jvi.44.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Cohen J., Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976 Apr;126(1):177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Davidoff-Abelson R. The characterization of a 120 S particle formed during phi 6 infection. Virology. 1980 Jun;103(2):386–391. doi: 10.1016/0042-6822(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala P. M., Romantschuk M., Bamford D. H. Purified phi 6 nucleocapsids are capable of productive infection of host cells with partially disrupted outer membranes. Virology. 1990 Oct;178(2):364–372. doi: 10.1016/0042-6822(90)90333-m. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Gottlieb P., Strassman J., Qiao X. Y., Bamford D. H., Mindich L. In vitro assembly of infectious nucleocapsids of bacteriophage phi 6: formation of a recombinant double-stranded RNA virus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9173–9177. doi: 10.1073/pnas.87.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantschuk M., Bamford D. H. Function of pili in bacteriophage phi 6 penetration. J Gen Virol. 1985 Nov;66(Pt 11):2461–2469. doi: 10.1099/0022-1317-66-11-2461. [DOI] [PubMed] [Google Scholar]
- Romantschuk M., Bamford D. H. phi 6-resistant phage-producing mutants of Pseudomonas phaseolicola. J Gen Virol. 1981 Oct;56(Pt 2):287–295. doi: 10.1099/0022-1317-56-2-287. [DOI] [PubMed] [Google Scholar]
- Romantschuk M., Olkkonen V. M., Bamford D. H. The nucleocapsid of bacteriophage phi 6 penetrates the host cytoplasmic membrane. EMBO J. 1988 Jun;7(6):1821–1829. doi: 10.1002/j.1460-2075.1988.tb03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Mindich L. RNA synthesis during infection with bacteriophage phi6. Virology. 1976 Nov;75(1):209–217. doi: 10.1016/0042-6822(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Tzagoloff A., Levine D., Mindich L. Proteins of bacteriophage phi6. J Virol. 1975 Sep;16(3):685–695. doi: 10.1128/jvi.16.3.685-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]