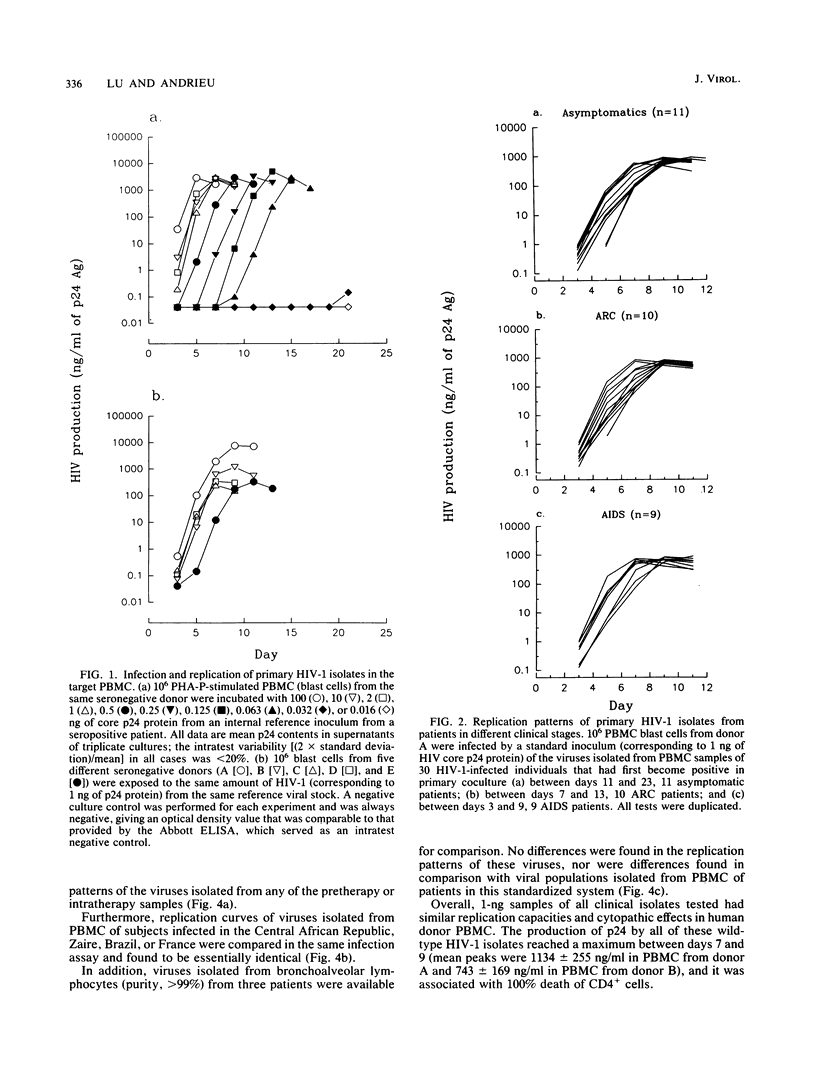
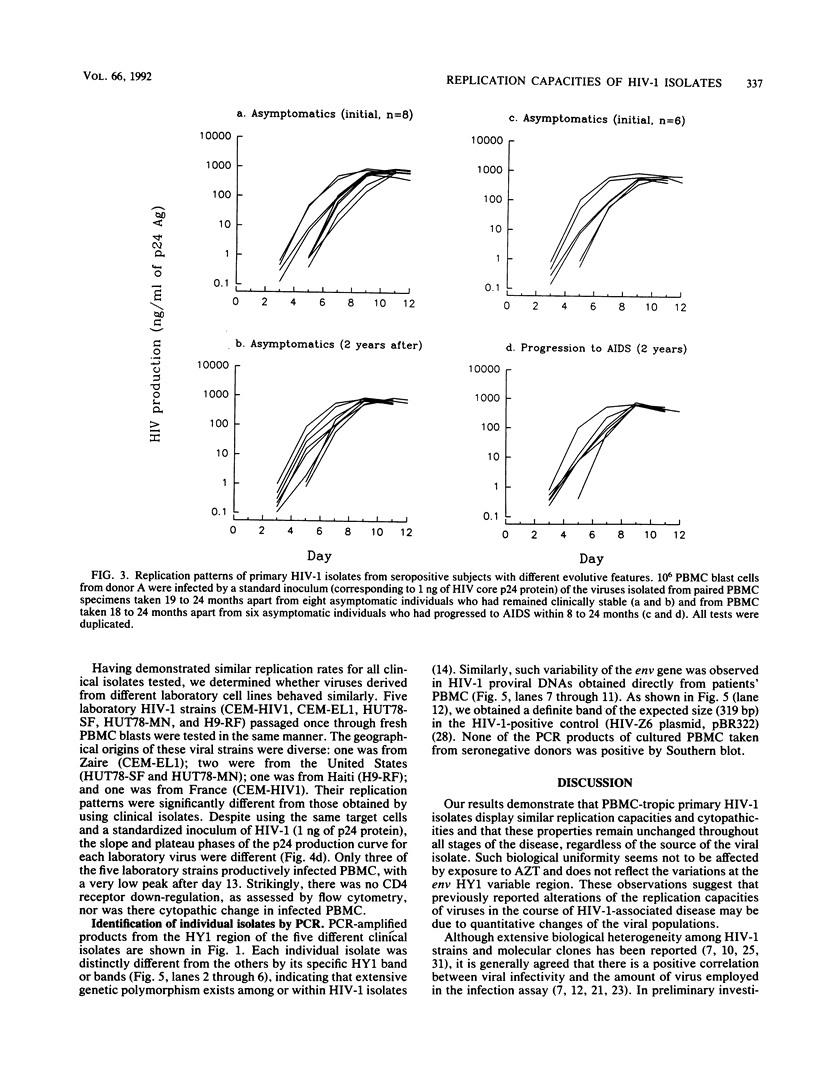
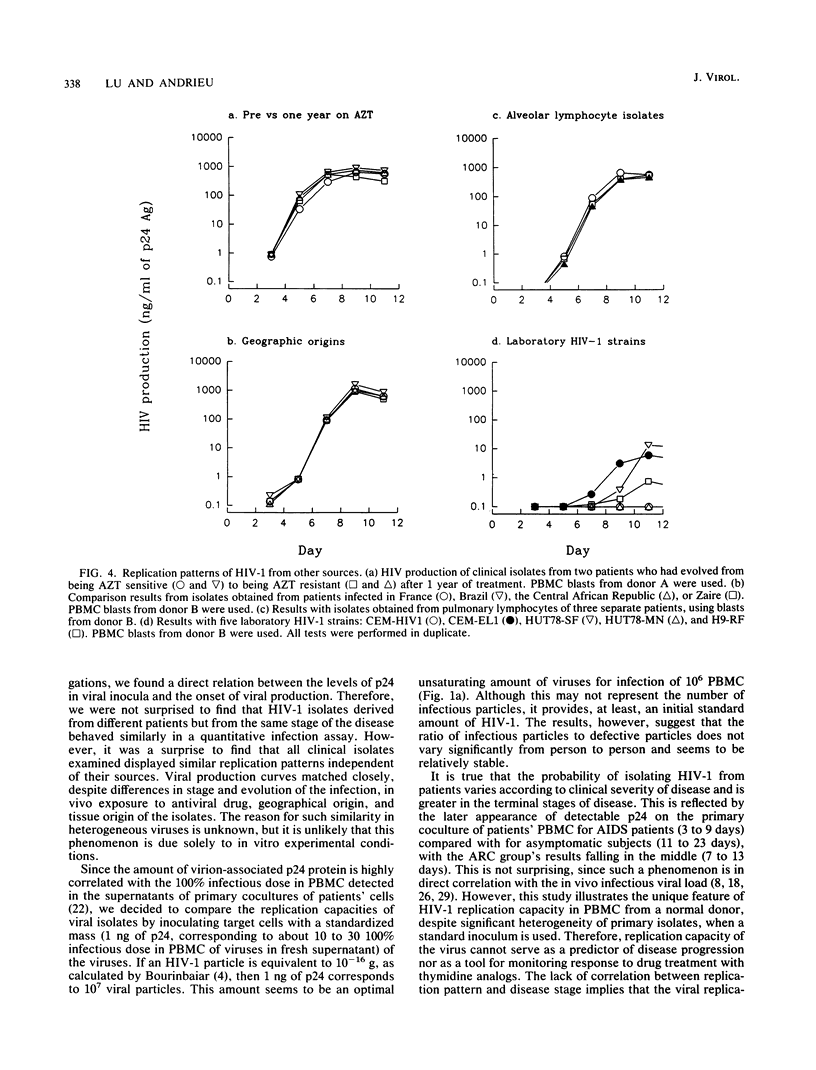
Abstract
Numerous studies have suggested that there are significant differences in replication capacities and cytopathicities among human immunodeficiency virus type 1 (HIV-1) isolates and that these differences correlate with the clinical status and geographical origin of infected individuals. However, it has been difficult to assess whether reported distinctions could be attributed to the methods used or whether they imply a true disparity between viral isolates. We thus attempted to characterize the replication properties of HIV-1 isolates directly recovered from infected patients (primary isolates) by using a standardized infection assay. Viruses were isolated from patients' peripheral blood mononuclear cells (PBMC) by a single coculture with normal donor PBMC stimulated with phytohemagglutinin. Replication curves and cytopathic effect of a standard inoculum (1 ng of p24) of 66 primary HIV-1 isolates were similar regardless of clinical stage of the patient (asymptomatic, AIDS-related complex, or AIDS) and evolutive feature (rate of progression to AIDS). There was no difference between viruses derived from patients sensitive to zidovudine and those derived from patients resistant to zidovudine. Moreover, no difference was found among viral isolates of different geographical origins (Central Africa, Zaire, Brazil, or France). Similarly, the replication patterns and cytopathicities of isolates from bronchoalveolar lymphocytes did not differ from those of isolates derived from PBMC. In contrast, the same amount of viral inoculum of five laboratory HIV-1 strains (HIV-1, EL1, SF, MN, and RF) produced different replication curves and were much less cytopathic. In contrast to laboratory viral strains, it appears that the primary HIV-1 isolates tested, whatever their clinical status and source, exhibited similar replication capacities and cytopathicities in allogeneic donor PBMC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Bourinbaiar A. S. HIV and gag. Nature. 1991 Jan 10;349(6305):111–111. doi: 10.1038/349111b0. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Saag M. S., Decker W. D., Campbell-Hill S., Roberson J. L., Veldkamp P. J., Kappes J. C., Hahn B. H., Shaw G. M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991 Apr 4;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Moore B. E. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology. 1990 Jan;174(1):103–116. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus S., Cheynier R., Wain-Hobson S. Evolution of human immunodeficiency virus type 1 nef and long terminal repeat sequences over 4 years in vivo and in vitro. J Virol. 1991 Jan;65(1):225–231. doi: 10.1128/jvi.65.1.225-231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca J., Wang R. Y., Alter H. J., Shih J. W. Clinical correlation and genetic polymorphism of the human immunodeficiency virus proviral DNA obtained after polymerase chain reaction amplification. J Infect Dis. 1990 Nov;162(5):1025–1030. doi: 10.1093/infdis/162.5.1025. [DOI] [PubMed] [Google Scholar]
- Goodenow M., Huet T., Saurin W., Kwok S., Sninsky J., Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Spouge J. L., Dembo M. Quantifying the infectivity of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4644–4648. doi: 10.1073/pnas.86.12.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari F. E., Poli G., Schnittman S. M., Psallidopoulos M. C., Davey V., Fauci A. S. In vivo T lymphocyte origin of macrophage-tropic strains of HIV. Role of monocytes during in vitro isolation and in vivo infection. J Immunol. 1990 Jun 15;144(12):4628–4632. [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Kennedy M. S., Cabridilla C. D., Feorino P. M., Francis D. P., Hicks D., Kalyanaraman V. S., Martin L. S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods. 1985 Jan 21;76(1):171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- Sakai K., Dewhurst S., Ma X. Y., Volsky D. J. Differences in cytopathogenicity and host cell range among infectious molecular clones of human immunodeficiency virus type 1 simultaneously isolated from an individual. J Virol. 1988 Nov;62(11):4078–4085. doi: 10.1128/jvi.62.11.4078-4085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Greenhouse J. J., Psallidopoulos M. C., Baseler M., Salzman N. P., Fauci A. S., Lane H. C. Increasing viral burden in CD4+ T cells from patients with human immunodeficiency virus (HIV) infection reflects rapidly progressive immunosuppression and clinical disease. Ann Intern Med. 1990 Sep 15;113(6):438–443. doi: 10.7326/0003-4819-113-6-438. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Fenyö E. M., Pavlakis G. N. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7200–7203. doi: 10.1073/pnas.86.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Anand R., York D., Ranganathan P., Feorino P., Schochetman G., Curran J., Kalyanaraman V. S., Luciw P. A., Sanchez-Pescador R. Molecular characterization of human immunodeficiency virus from Zaire: nucleotide sequence analysis identifies conserved and variable domains in the envelope gene. Gene. 1987;52(1):71–82. doi: 10.1016/0378-1119(87)90396-9. [DOI] [PubMed] [Google Scholar]
- Vartanian J. P., Meyerhans A., Asjö B., Wain-Hobson S. Selection, recombination, and G----A hypermutation of human immunodeficiency virus type 1 genomes. J Virol. 1991 Apr;65(4):1779–1788. doi: 10.1128/jvi.65.4.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet A., Lu W., Beldjord K., Andrieu J. M. Correlation between CD4 cell counts and cellular and plasma viral load in HIV-1-seropositive individuals. AIDS. 1991 Mar;5(3):283–288. doi: 10.1097/00002030-199103000-00006. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]
- von Briesen H., Becker W. B., Henco K., Helm E. B., Gelderblom H. R., Brede H. D., Rübsamen-Waigmann H. Isolation frequency and growth properties of HIV-variants: multiple simultaneous variants in a patient demonstrated by molecular cloning. J Med Virol. 1987 Sep;23(1):51–66. doi: 10.1002/jmv.1890230107. [DOI] [PubMed] [Google Scholar]