Abstract
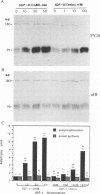
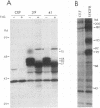
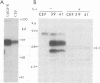
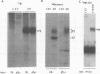
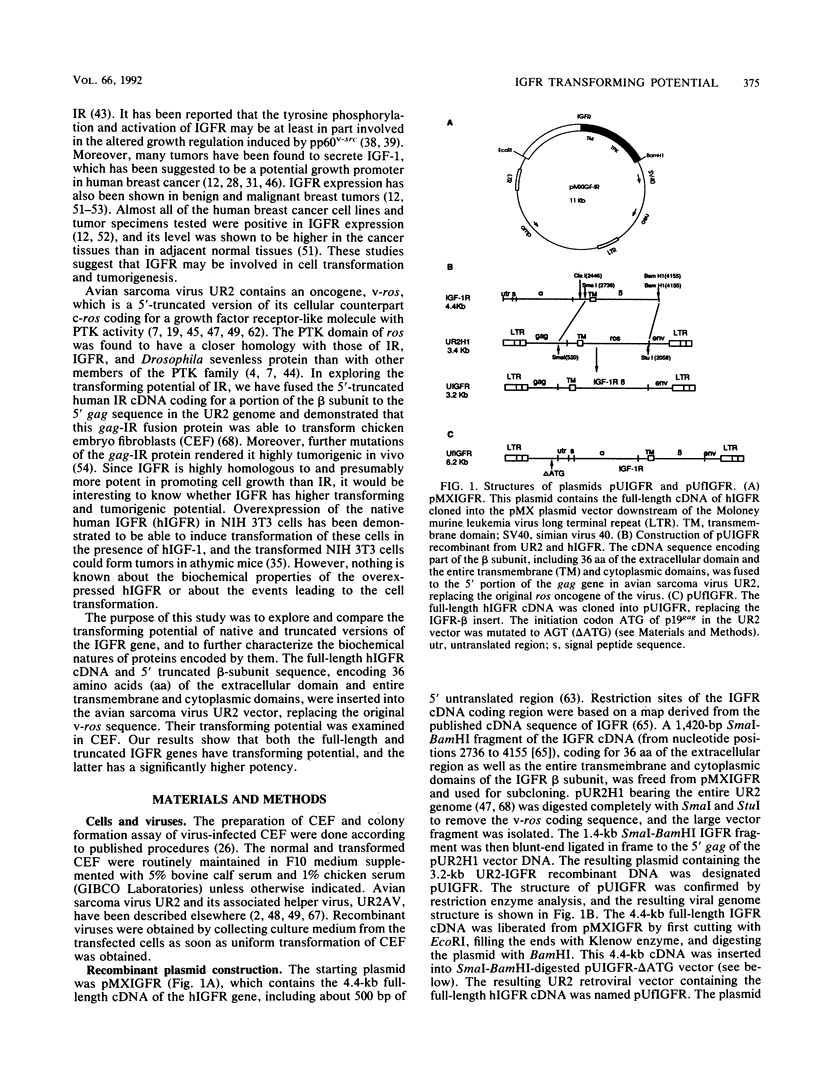
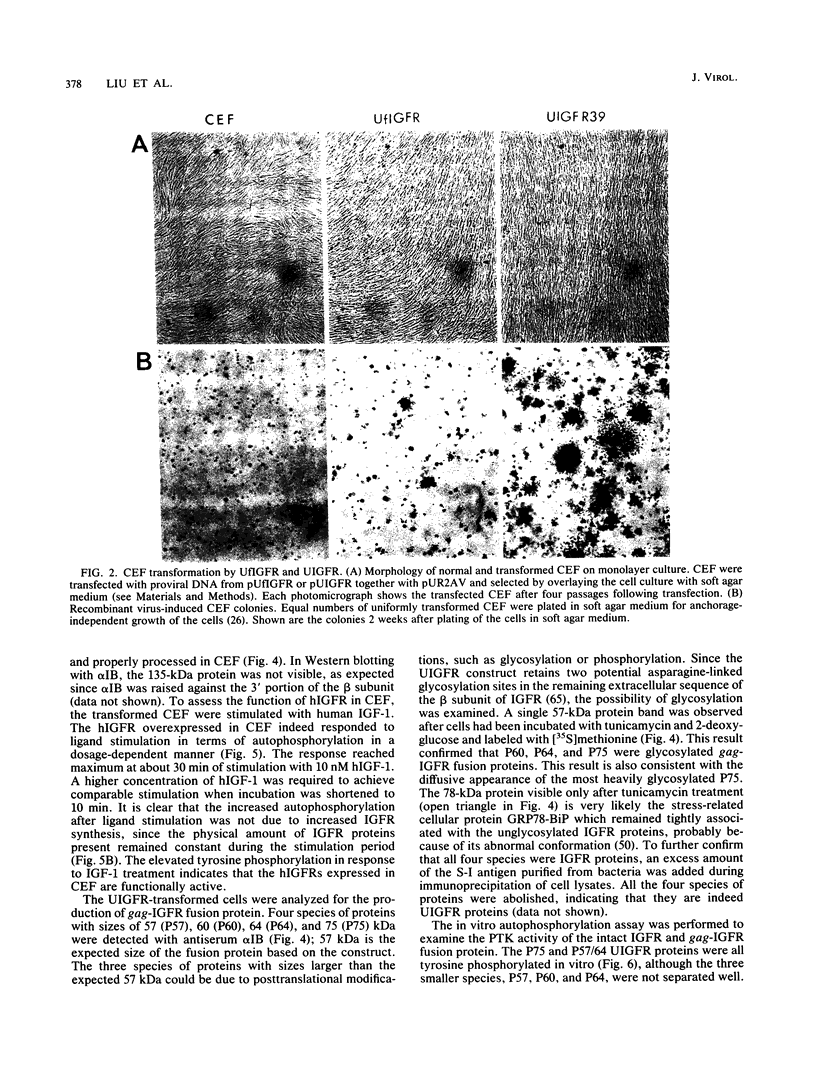
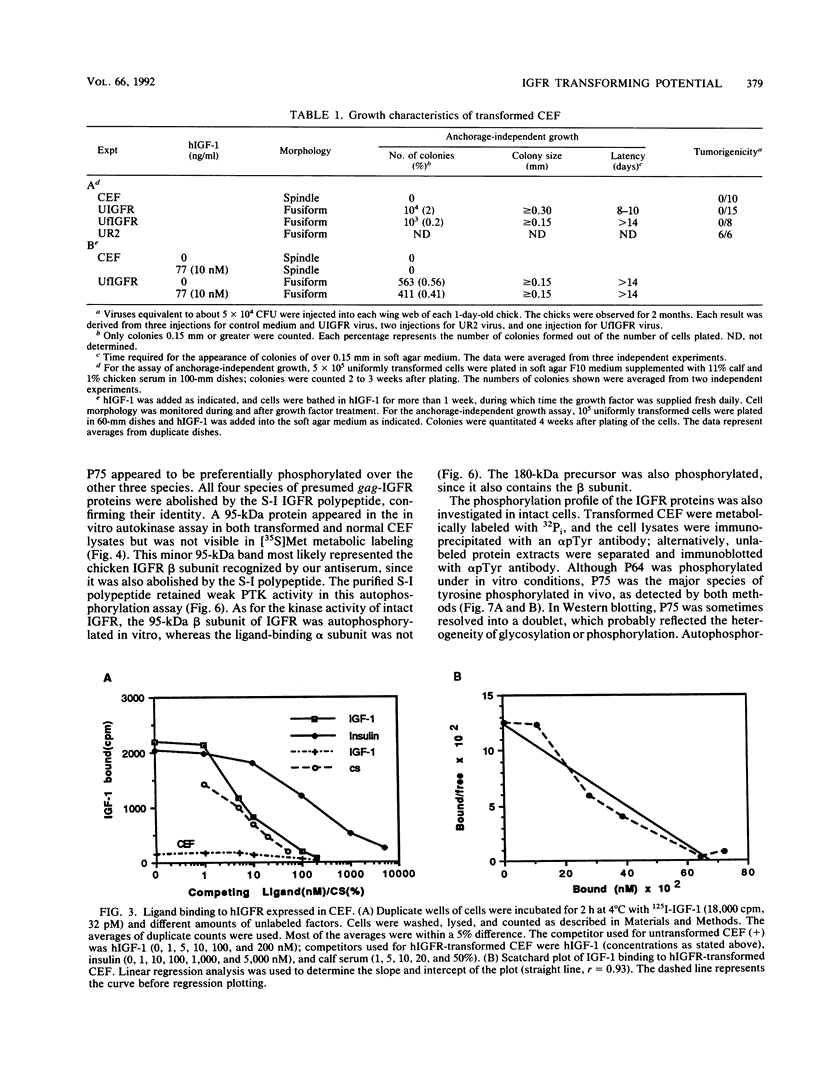
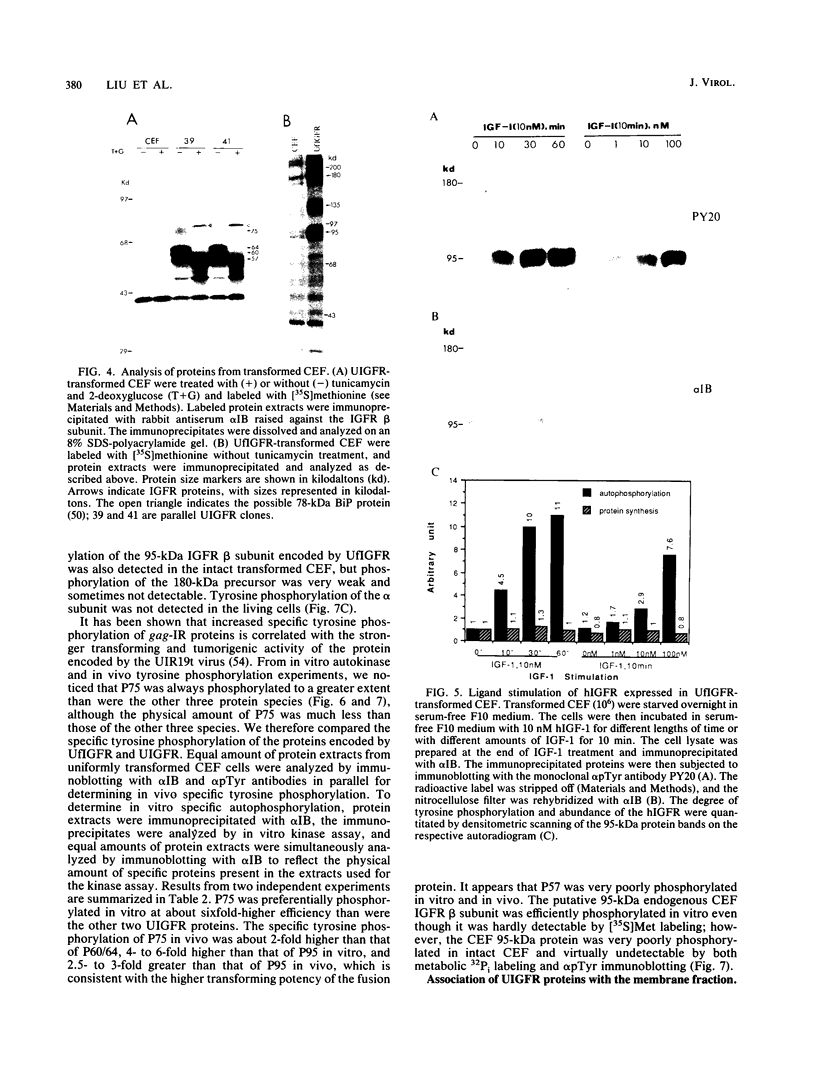
The human insulinlike growth factor 1 (hIGF-1) receptor (hIGFR) is a transmembrane protein tyrosine kinase (PTK) molecule which shares high sequence homology in the PTK domain with the insulin receptor and, to a lesser degree, the ros transforming protein of avian sarcoma virus UR2. To assess the transforming potential of hIGFR, we introduced the intact and altered hIGFR into chicken embryo fibroblasts (CEF). The full-length hIGFR cDNA (fIGFR) was cloned into a UR2 retroviral vector, replacing the original oncogene v-ros. fIGFR was able to promote the growth of CEF in soft agar and cause morphological alteration in the absence of added hIGF-1 to medium containing 11% calf and 1% chicken serum. The transforming ability of hIGFR was not further increased in the presence of 10 nM exogenous hIGF-1. The 180-kDa protein precursor of hIGFR was synthesized and processed into alpha and beta subunits. The overexpressed hIGFR in CEF bound hIGF-1 with high affinity (Kd = 5.4 x 10(-9) M) and responded to ligand stimulation with increased tyrosine autophosphorylation. The cDNA sequence coding for part of the beta subunit of hIGFR, including 36 amino acids of the extracellular domain and the entire transmembrane and cytoplasmic domains, was fused to the 5' portion of the gag gene in the UR2 vector to form an avian retrovirus. The resulting virus, named UIGFR, was able to induce morphological transformation and promote colony formation of CEF with a stronger potency than did fIGFR. The UIGFR genome encodes a membrane-associated, glycosylated gag-IGFR fusion protein. The specific tyrosine phosphorylation of the mature form of the fusion protein, P75, is sixfold higher in vitro and threefold higher in vivo than that of the native IGFR beta subunit, P95. In conclusion, overexpression of the native or an altered hIGFR can induce transformation of CEF with the gag-IGFR fusion protein possessing enhanced transforming potential, which is consistent with its increased in vitro and in vivo tyrosine phosphorylation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R. Secretory protein translocation in a neurospora crassa in vitro system. Hydrolysis of a nucleoside triphosphate is required for posttranslational translocation. J Biol Chem. 1987 Dec 15;262(35):17031–17037. [PubMed] [Google Scholar]
- Balduzzi P. C., Notter M. F., Morgan H. R., Shibuya M. Some biological properties of two new avian sarcoma viruses. J Virol. 1981 Oct;40(1):268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Johnson R. J., Owens P. C., Francis G. L., Upton F. M., McMurtry J. P., Wallace J. C. Chicken insulin-like growth factor-I: amino acid sequence, radioimmunoassay, and plasma levels between strains and during growth. Gen Comp Endocrinol. 1990 Sep;79(3):459–468. doi: 10.1016/0016-6480(90)90076-x. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., O'Neill K., Riggs M., Wigler M. Characterization of ROS1 cDNA from a human glioblastoma cell line. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4799–4803. doi: 10.1073/pnas.87.12.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Kaligian A., DelVecchio R., Pilch P. F. Ligand-dependent intersubunit association within the insulin receptor complex activates its intrinsic kinase activity. J Biol Chem. 1988 May 15;263(14):6822–6828. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. M., Heller D., Poon B., Kang L., Wang L. H. The proto-oncogene c-ros codes for a transmembrane tyrosine protein kinase sharing sequence and structural homology with sevenless protein of Drosophila melanogaster. Oncogene. 1991 Feb;6(2):257–264. [PubMed] [Google Scholar]
- Copeland N. G., Gilbert D. J., Cho B. C., Donovan P. J., Jenkins N. A., Cosman D., Anderson D., Lyman S. D., Williams D. E. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990 Oct 5;63(1):175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. J., Yee D., Sly W. S., Perdue J., Hampton B., Lippman M. E., Rosen N. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 1990 Jan 1;50(1):48–53. [PubMed] [Google Scholar]
- Czech M. P. Signal transmission by the insulin-like growth factors. Cell. 1989 Oct 20;59(2):235–238. doi: 10.1016/0092-8674(89)90281-x. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell. 1982 Nov;31(1):8–10. doi: 10.1016/0092-8674(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Kapadia M., Yanow C. E., Fabrick K., Mariz I. K. Insulin-like growth factors I and II of nonmammalian sera. Gen Comp Endocrinol. 1985 Aug;59(2):316–325. doi: 10.1016/0016-6480(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Debant A., Clauser E., Ponzio G., Filloux C., Auzan C., Contreres J. O., Rossi B. Replacement of insulin receptor tyrosine residues 1162 and 1163 does not alter the mitogenic effect of the hormone. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8032–8036. doi: 10.1073/pnas.85.21.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Hanafusa T., Hanafusa H. Membrane association of the transforming protein of avian sarcoma virus UR2 and mutants temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1985 Dec;56(3):790–797. doi: 10.1128/jvi.56.3.790-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Betsholtz C., Claesson-Welsh L., Westermark B. Subversion of growth regulatory pathways in malignant transformation. Biochim Biophys Acta. 1987 Nov 25;907(3):219–244. doi: 10.1016/0304-419x(87)90007-2. [DOI] [PubMed] [Google Scholar]
- Huang E., Nocka K., Beier D. R., Chu T. Y., Buck J., Lahm H. W., Wellner D., Leder P., Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990 Oct 5;63(1):225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Huff K. K., Kaufman D., Gabbay K. H., Spencer E. M., Lippman M. E., Dickson R. B. Secretion of an insulin-like growth factor-I-related protein by human breast cancer cells. Cancer Res. 1986 Sep;46(9):4613–4619. [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Jaques G., Rotsch M., Wegmann C., Worsch U., Maasberg M., Havemann K. Production of immunoreactive insulin-like growth factor I and response to exogenous IGF-I in small cell lung cancer cell lines. Exp Cell Res. 1988 Jun;176(2):336–343. doi: 10.1016/0014-4827(88)90335-7. [DOI] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Role of gag sequence in the biochemical properties and transforming activity of the avian sarcoma virus UR2-encoded gag-ros fusion protein. J Virol. 1990 Dec;64(12):5997–6009. doi: 10.1128/jvi.64.12.5997-6009.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. The transforming protein P68gag-ros of avian sarcoma virus UR2 is a transmembrane protein with the gag portion protruding extracellularly. Oncogene Res. 1987 Jun;1(1):7–21. [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Two point mutations in the transmembrane domain of P68gag-ros inactive its transforming activity and cause a delay in membrane association. J Virol. 1991 Jan;65(1):180–189. doi: 10.1128/jvi.65.1.180-189.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma L. M., Reynolds A. B., Weber M. J. Glycoprotein tyrosine phosphorylation in Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1990 Feb;10(2):837–841. doi: 10.1128/mcb.10.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma L. M., Weber M. J. Constitutive phosphorylation of the receptor for insulinlike growth factor I in cells transformed by the src oncogene. Mol Cell Biol. 1990 Jul;10(7):3626–3634. doi: 10.1128/mcb.10.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R., Hanafusa H. Changes in amino-terminal sequences of pp60src lead to decreased membrane association and decreased in vivo tumorigenicity. Cell. 1982 Apr;28(4):889–896. doi: 10.1016/0092-8674(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Shibuya M. Tissue-specific expression of rat c-ros-1 gene and partial structural similarity of its predicted products with sev protein of Drosophila melanogaster. J Virol. 1990 May;64(5):2117–2125. doi: 10.1128/jvi.64.5.2117-2125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Wang L. H., Shibuya M. Human c-ros-1 gene homologous to the v-ros sequence of UR2 sarcoma virus encodes for a transmembrane receptorlike molecule. Mol Cell Biol. 1986 Aug;6(8):3000–3004. doi: 10.1128/mcb.6.8.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuto F., Del Monte P., Barreca A., Fortini P., Cariola G., Catrambone G., Giordano G. Evidence for an increased somatomedin-C/insulin-like growth factor I content in primary human lung tumors. Cancer Res. 1986 Feb;46(2):985–988. [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Molecular cloning and characterization of avian sarcoma virus UR2 and comparison of its transforming sequence with those of other avian sarcoma viruses. J Virol. 1984 Jun;50(3):914–921. doi: 10.1128/jvi.50.3.914-921.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D. T., Hiebert S. W., Lamb R. A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990 May;10(5):1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen F., Partanen S., Mäkinen T., Rutanen E. M. Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res. 1988 Mar 1;48(5):1343–1347. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Beuscart R., Djiane J., Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer Res. 1988 Nov 15;48(22):6429–6433. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Laurent J. C., Louchez M. M., Amrani S., Leroy-Martin B., Vilain M. O., Delobelle A., Demaille A. Presence and characterization of insulin-like growth factor 1 receptors in human benign breast disease. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1425–1431. doi: 10.1016/0277-5379(88)90332-x. [DOI] [PubMed] [Google Scholar]
- Poon B., Dixon D., Ellis L., Roth R. A., Rutter W. J., Wang L. H. Molecular basis of the activation of the tumorigenic potential of Gag-insulin receptor chimeras. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):877–881. doi: 10.1073/pnas.88.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read L. C., Ballard F. J., Francis G. L., Baxter R. C., Bagley C. J., Wallace J. C. Comparative binding of bovine, human and rat insulin-like growth factors to membrane receptors and to antibodies against human insulin-like growth factor-1. Biochem J. 1986 Jan 1;233(1):215–221. doi: 10.1042/bj2330215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P., Roth J. Hormonal regulation of human growth. N Engl J Med. 1987 Apr 9;316(15):941–943. doi: 10.1056/NEJM198704093161510. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. Stimulation of tyrosine-specific phosphorylation in vitro by insulin-like growth factor I. 1983 Sep 29-Oct 5Nature. 305(5933):438–440. doi: 10.1038/305438a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sharma S., Birchmeier C., Nikawa J., O'Neill K., Rodgers L., Wigler M. Characterization of the ros1-gene products expressed in human glioblastoma cell lines. Oncogene Res. 1989;5(2):91–100. [PubMed] [Google Scholar]
- Steele-Perkins G., Turner J., Edman J. C., Hari J., Pierce S. B., Stover C., Rutter W. J., Roth R. A. Expression and characterization of a functional human insulin-like growth factor I receptor. J Biol Chem. 1988 Aug 15;263(23):11486–11492. [PubMed] [Google Scholar]
- Tornqvist H. E., Pierce M. W., Frackelton A. R., Nemenoff R. A., Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987 Jul 25;262(21):10212–10219. [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure and transforming sequence of avian sarcoma virus UR2. J Virol. 1982 Mar;41(3):833–841. doi: 10.1128/jvi.41.3.833-841.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Lin B., Jong S. M., Dixon D., Ellis L., Roth R. A., Rutter W. J. Activation of transforming potential of the human insulin receptor gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5725–5729. doi: 10.1073/pnas.84.16.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]