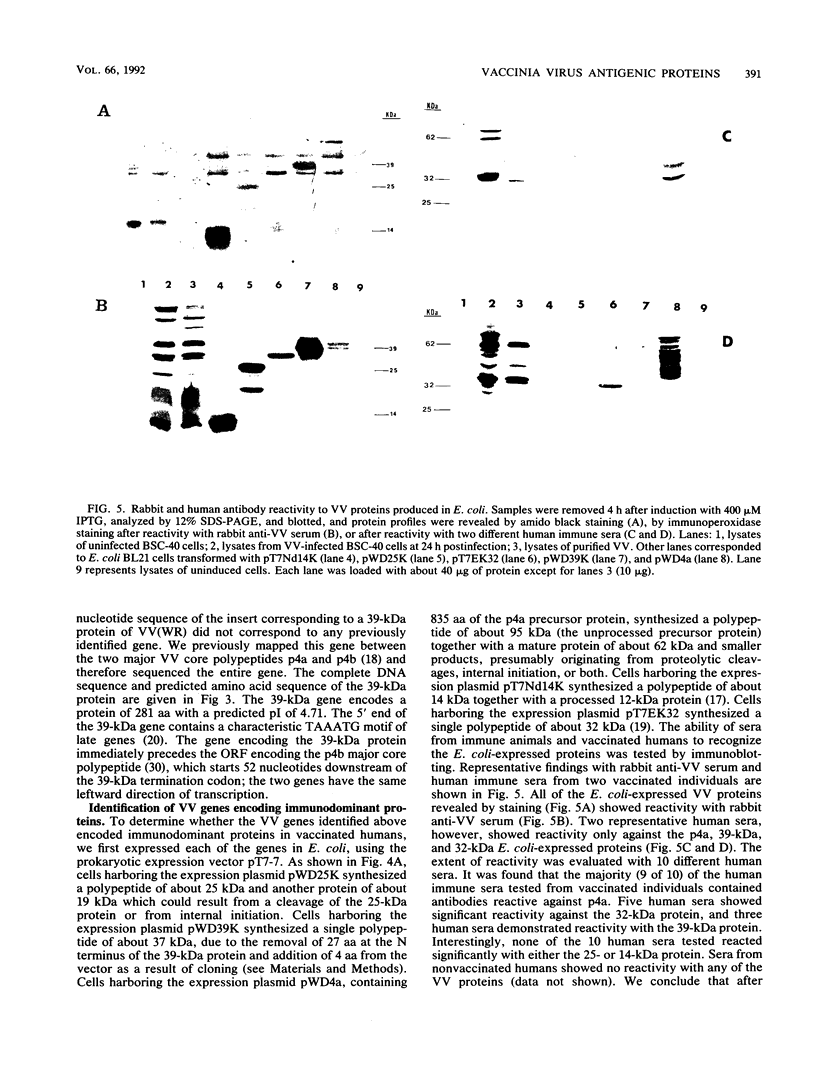
Abstract
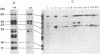
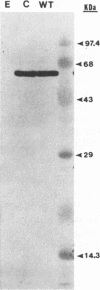
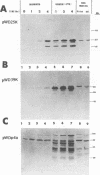
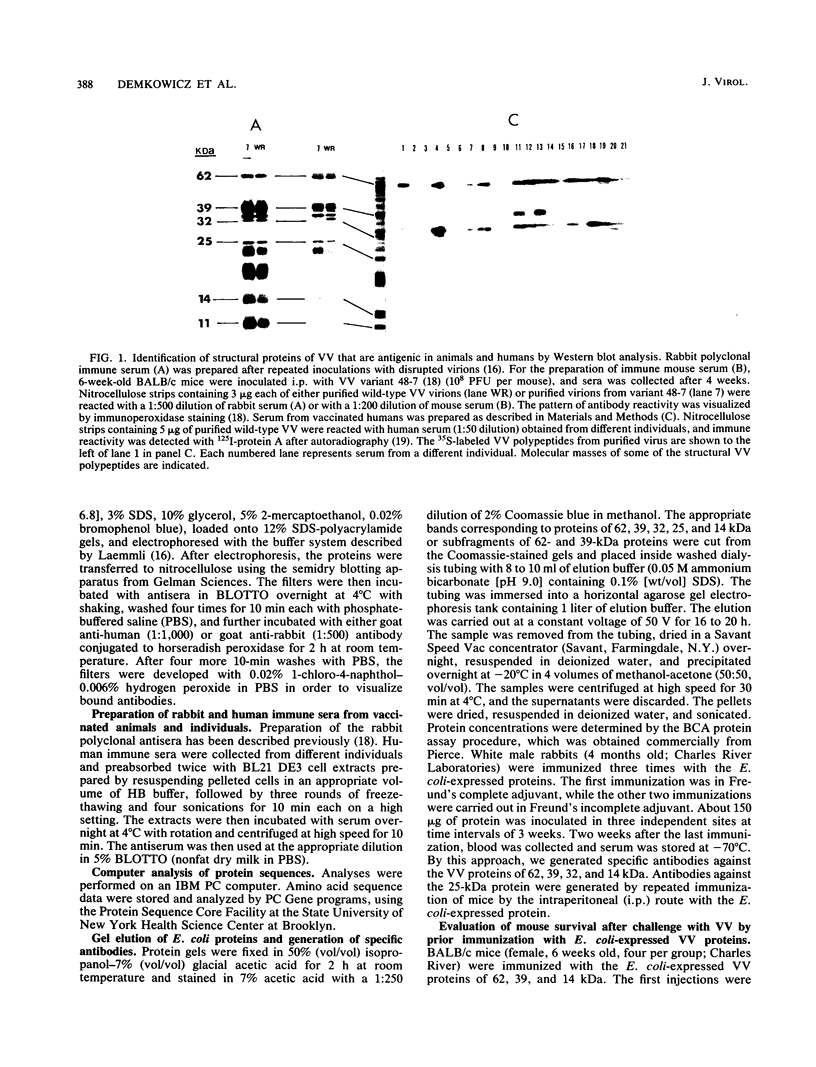
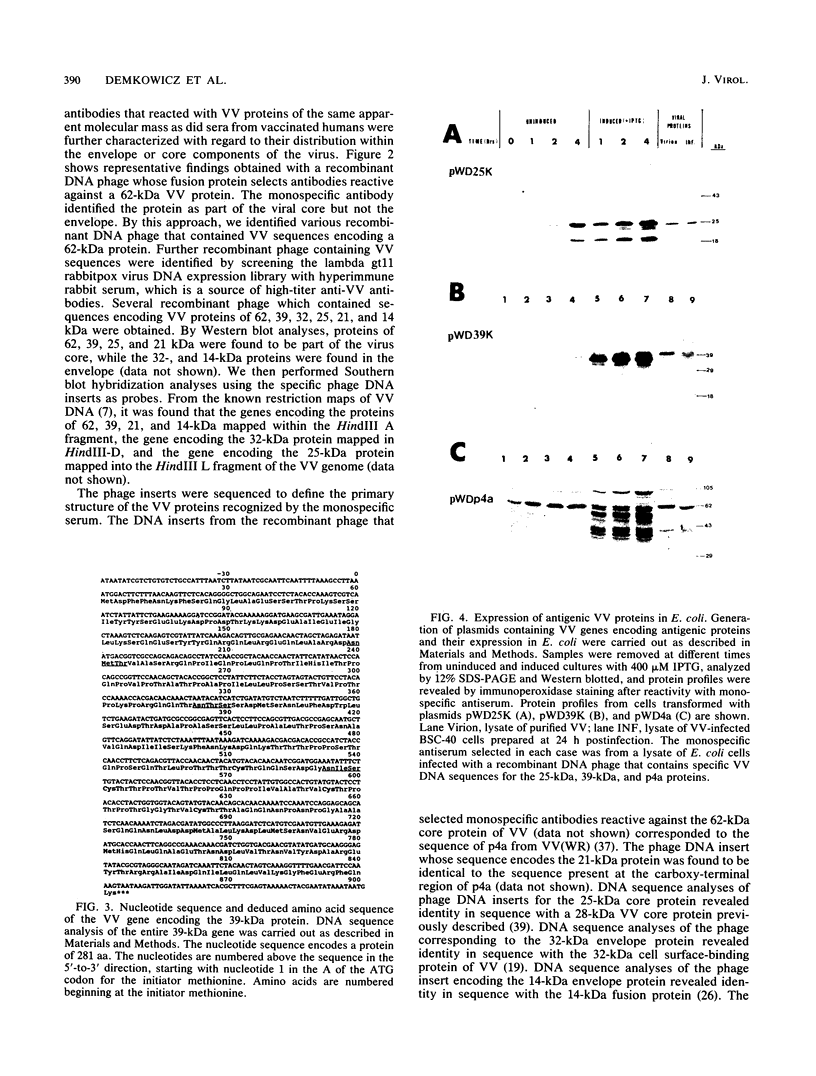
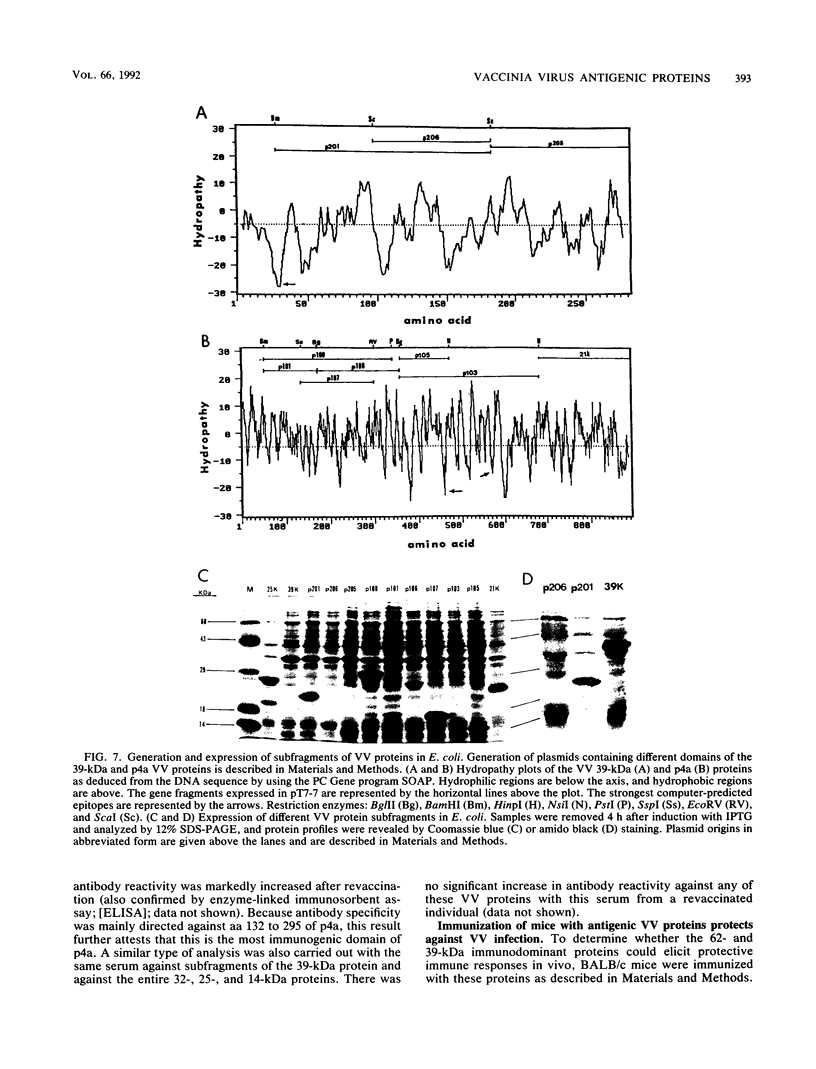
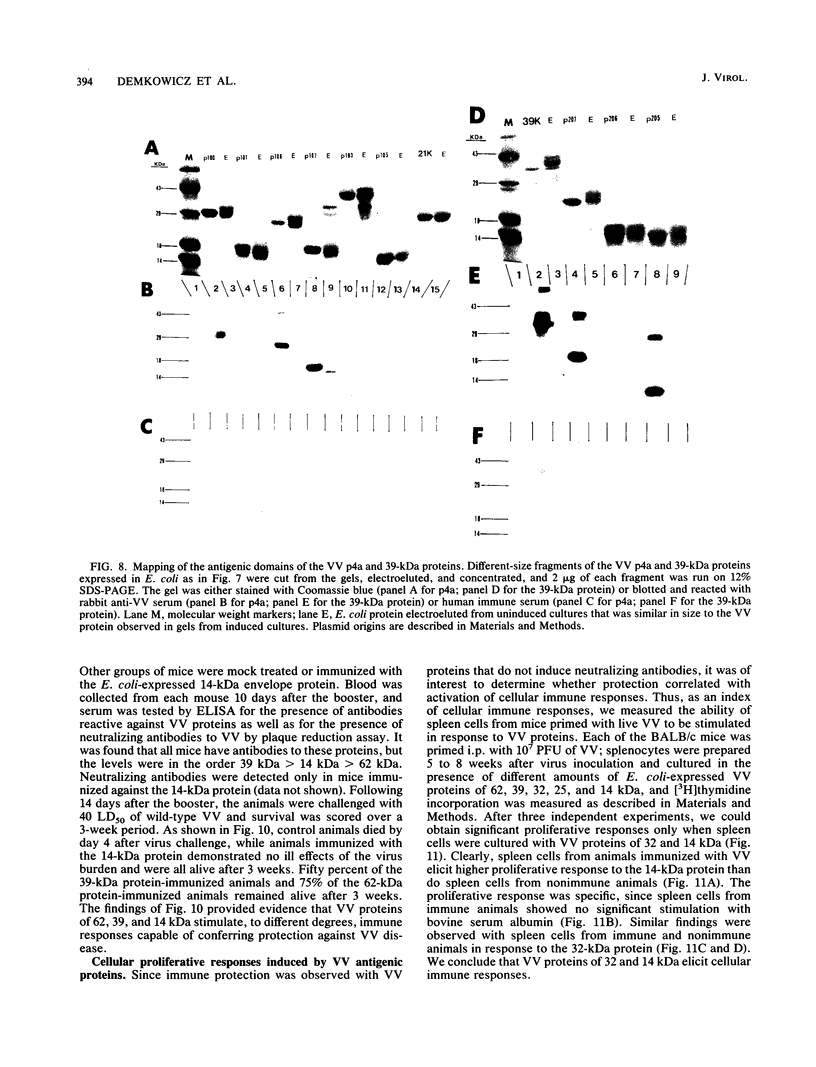
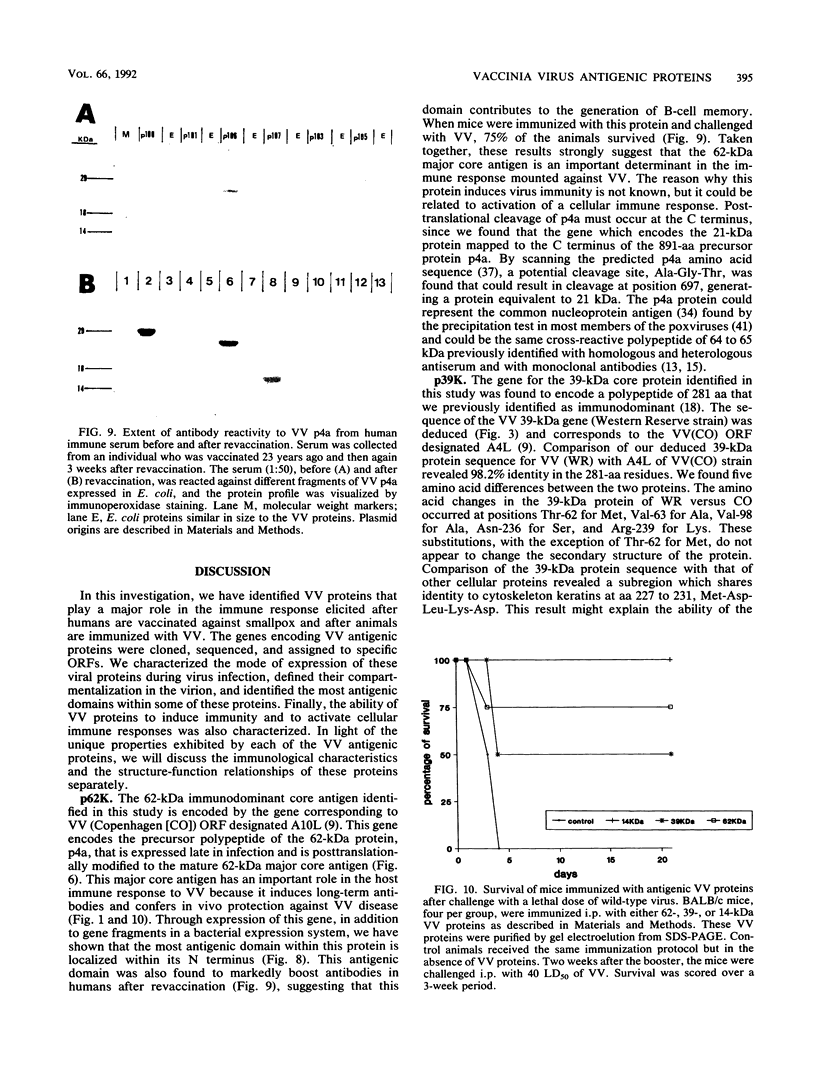
Vaccinia virus (VV) is a potent immunogen, but the nature of VV proteins involved in the activation of the immune response of the host is not yet known. By screening a lambda gt11 expression library of rabbitpox virus DNA with serum from humans vaccinated against smallpox or with serum from VV-immunized animals, we identified several VV genes that encode highly antigenic viral proteins with molecular masses of 62, 39, 32, 25, 21, and 14 kDa. It was found that VV proteins of 62, 39, 25, and 21 kDa are part of the virus core, while proteins of 32 and 14 kDa are part of the virus envelope. All of these proteins were synthesized at late times postinfection. Proteins of 62 and 25 kDa were produced by cleavage of larger precursors of 95 kDa (p4a) and 28 kDa, respectively. The 21-kDa protein was the result of a cleavage of p4a, presumably at amino acid Gly-697. DNA sequence analysis, in comparison with the known nucleotide sequence of VV, provided identification of the corresponding open reading frames. Expression of the viral genes in Escherichia coli was used to monitor which of the viral antigens elicit immunodominant responses and the location of antigenic domains. Three viral antigens of 62, 39, and 32 kDa exhibited immunodominant characteristics. The most antigenic sites of 62 and 39 kDa were identified at the N terminus (amino acids 132 to 295) and C terminus (last 103 amino acids), respectively. Immunization of mice with the 62-, 39-, or 14-kDa antigenic proteins conferred different degrees of protection from VV challenge. Proteins of 32 and 14 kDa induced cellular proliferative responses in VV-infected mice. Our findings demonstrate the nature of VV proteins involved in the activation of host immune responses after vaccination, provide identification of the viral gene locus, and define structural and immunological properties of these antigenic VV proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Binns M., Mason C., Boursnell M. A 39,000 Mr immunodominant protein of fowlpox virus contains multiple copies of a 12 amino acid repeat sequence. J Gen Virol. 1990 Dec;71(Pt 12):2883–2888. doi: 10.1099/0022-1317-71-12-2883. [DOI] [PubMed] [Google Scholar]
- Chain B., McCafferty I., Wallace G., Askenase P. W. Improvement of the in vitro T cell proliferation assay by a modified method that separates the antigen recognition and IL-2-dependent steps. J Immunol Methods. 1987 May 20;99(2):221–228. doi: 10.1016/0022-1759(87)90131-1. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Similarity of vaccinia 28K, v-erb-B and EGF receptors. Nature. 1985 Jul 18;316(6025):219–220. doi: 10.1038/316219b0. [DOI] [PubMed] [Google Scholar]
- Czerny C. P., Mahnel H. Structural and functional analysis of orthopoxvirus epitopes with neutralizing monoclonal antibodies. J Gen Virol. 1990 Oct;71(Pt 10):2341–2352. doi: 10.1099/0022-1317-71-10-2341. [DOI] [PubMed] [Google Scholar]
- Dallo S., Maa J. S., Rodriguez J. R., Rodriguez D., Esteban M. Humoral immune response elicited by highly attenuated variants of vaccinia virus and by an attenuated recombinant expressing HIV-1 envelope protein. Virology. 1989 Nov;173(1):323–329. doi: 10.1016/0042-6822(89)90250-x. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S. C., Lai C. F., Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990 Sep;178(1):81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- Gordon J., Kovala T., Dales S. Molecular characterization of a prominent antigen of the vaccinia virus envelope. Virology. 1988 Dec;167(2):361–369. [PubMed] [Google Scholar]
- Hirt P., Hiller G., Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986 Jun;58(3):757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Miyamoto H., Kato S. Serologically cross-reactive polypeptides in vaccinia, cowpox and Shope fibroma viruses. J Gen Virol. 1979 Aug;44(2):557–563. doi: 10.1099/0022-1317-44-2-557. [DOI] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto N., Tanimoto S., Hiroi K., Ozaki M., Miyamoto H., Wakamiya N., Ikuta K., Ueda S., Kato S. Monoclonal antibodies to cowpox virus: polypeptide analysis of several major antigens. J Gen Virol. 1987 Jan;68(Pt 1):239–246. doi: 10.1099/0022-1317-68-1-239. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. F., Gong S. C., Esteban M. Structural and functional properties of the 14-kDa envelope protein of vaccinia virus synthesized in Escherichia coli. J Biol Chem. 1990 Dec 25;265(36):22174–22180. [PubMed] [Google Scholar]
- Lai C. F., Gong S. C., Esteban M. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J Virol. 1991 Oct;65(10):5631–5635. doi: 10.1128/jvi.65.10.5631-5635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa J. S., Esteban M. Structural and functional studies of a 39,000-Mr immunodominant protein of vaccinia virus. J Virol. 1987 Dec;61(12):3910–3919. doi: 10.1128/jvi.61.12.3910-3919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa J. S., Rodriguez J. F., Esteban M. Structural and functional characterization of a cell surface binding protein of vaccinia virus. J Biol Chem. 1990 Jan 25;265(3):1569–1577. [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Seto J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J Virol. 1988 Oct;62(10):3772–3778. doi: 10.1128/jvi.62.10.3772-3778.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre F. J., Raska K., Jr, Holowczak J. A. The immune response to vaccinia virus infection in mice: analysis of the role of antibody. Arch Virol. 1989;107(3-4):273–289. doi: 10.1007/BF01317923. [DOI] [PubMed] [Google Scholar]
- Oie M., Ichihashi Y. Modification of vaccinia virus penetration proteins analyzed by monoclonal antibodies. Virology. 1987 Apr;157(2):449–459. doi: 10.1016/0042-6822(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Piccini A., Paoletti E. Vaccinia: virus, vector, vaccine. Adv Virus Res. 1988;34:43–64. doi: 10.1016/s0065-3527(08)60515-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987 Nov;61(11):3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. R., Rodriguez D., Esteban M. Insertional inactivation of the vaccinia virus 32-kilodalton gene is associated with attenuation in mice and reduction of viral gene expression in polarized epithelial cells. J Virol. 1992 Jan;66(1):183–189. doi: 10.1128/jvi.66.1.183-189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. R., Rodriguez D., Esteban M. Structural properties of HIV-1 Env fused with the 14-kDa vaccinia virus envelope protein. Virology. 1991 Apr;181(2):742–748. doi: 10.1016/0042-6822(91)90910-4. [DOI] [PubMed] [Google Scholar]
- Rosel J., Moss B. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J Virol. 1985 Dec;56(3):830–838. doi: 10.1128/jvi.56.3.830-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H. Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology. 1986 Apr 30;150(2):451–462. doi: 10.1016/0042-6822(86)90309-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meir E., Wittek R. Fine structure of the vaccinia virus gene encoding the precursor of the major core protein 4 a. Arch Virol. 1988;102(1-2):19–27. doi: 10.1007/BF01315559. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Hruby D. E. Posttranslational modification of vaccinia virus proteins. Curr Top Microbiol Immunol. 1990;163:185–206. doi: 10.1007/978-3-642-75605-4_7. [DOI] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. Serological relationships within the poxvirus group: an antigen common to all members of the group. Virology. 1962 Mar;16:334–341. doi: 10.1016/0042-6822(62)90255-6. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Use of a bacterial expression vector to identify the gene encoding a major core protein of vaccinia virus. J Virol. 1985 Nov;56(2):534–540. doi: 10.1128/jvi.56.2.534-540.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton S., Gordon J., Dales S. Identification of antigenic determinants by polyclonal and hybridoma antibodies induced during the course of infection by vaccinia virus. Virology. 1986 Jan 15;148(1):84–96. doi: 10.1016/0042-6822(86)90405-8. [DOI] [PubMed] [Google Scholar]
- Yang W. P., Kao S. Y., Bauer W. R. Biosynthesis and post-translational cleavage of vaccinia virus structural protein VP8. Virology. 1988 Dec;167(2):585–590. [PubMed] [Google Scholar]