Abstract
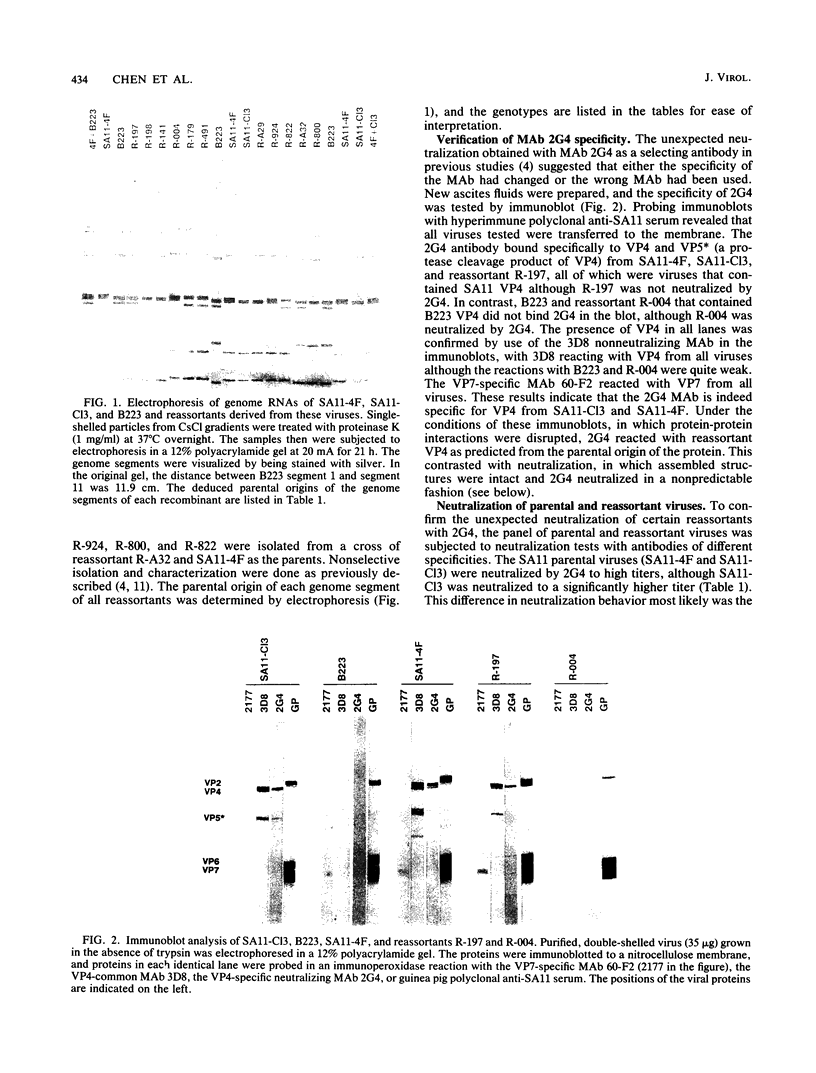
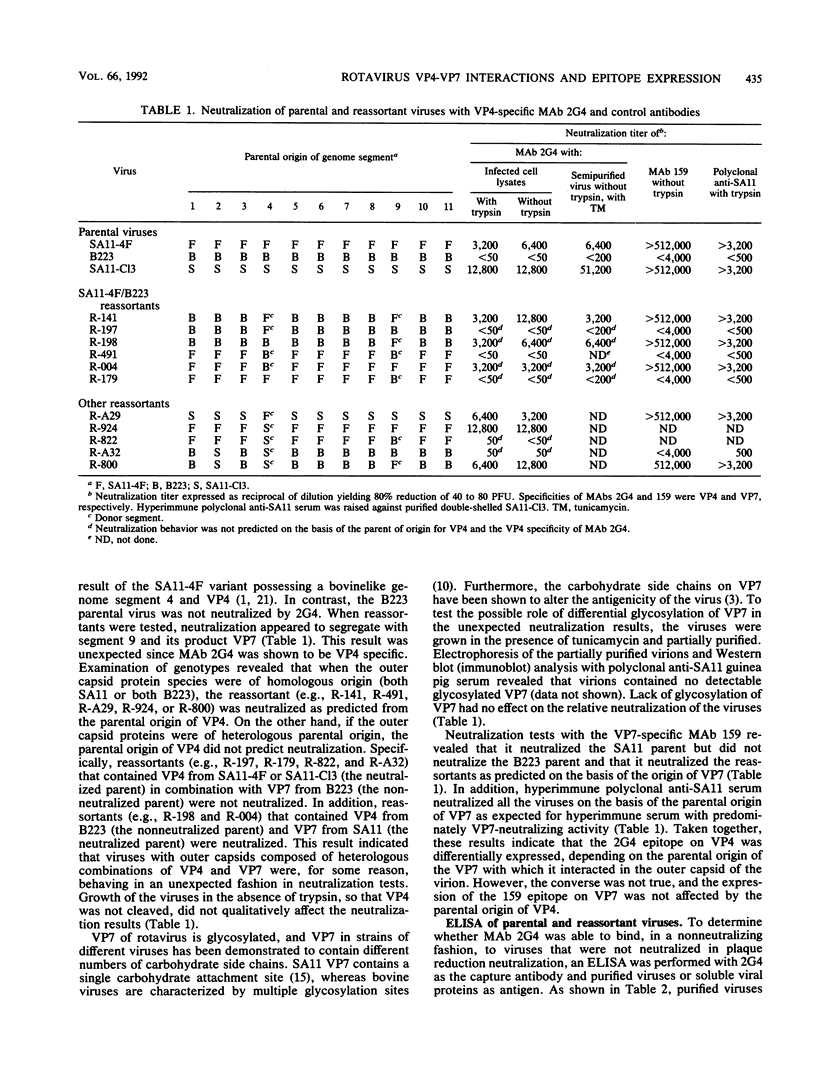
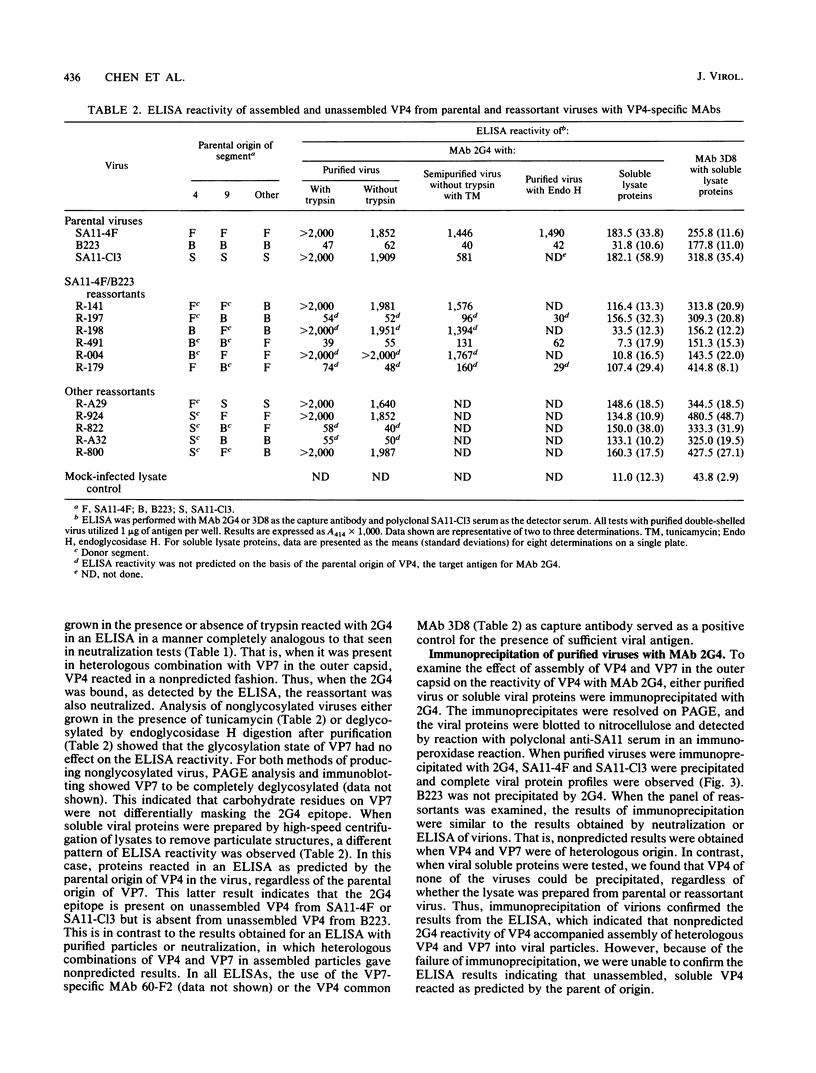
We previously reported that the expression of rotavirus phenotypes by reassortants was affected by recipient genetic background and proposed specific interactions between the outer capsid proteins VP4 and VP7 as the basis for the phenotypic effects (D. Chen, J. W. Burns, M. K. Estes, and R. F. Ramig, Proc. Natl. Acad. Sci. USA 86:3743-3747, 1989). A neutralizing, cross-reactive VP4-specific monoclonal antibody (MAb), 2G4, was used to probe the protein-protein interactions. The VP4 specificity of 2G4 was confirmed by immunoblot analysis. MAb 2G4 reacted with both standard (SA11-C13) and variant rotavirus SA11 (SA11-4F) but did not react with bovine rotavirus B223 as determined by plaque reduction neutralization (PRN) and enzyme-linked immunosorbent assay (ELISA). When a panel of SA11-4F/B223 and SA11-Cl3/B223 reassortants in purified or crude lysate form that had been grown in the presence or absence of trypsin was analyzed with MAb 2G4 by PRN and ELISA, the results with some reassortants were unexpected. That is, MAb 2G4 reacted with VP4 of SA11 parental origin (4F or C13) when it was assembled into capsids with the homologous SA11 VP7 but failed to react with VP4 of SA11 assembled into capsids with heterologous B223 VP7. Conversely, MAb 2G4 failed to react with VP4 of B223 parental origin when it was assembled into capsids with homologous B223 VP7 but did react with B223 VP4 assembled into capsids with the heterologous SA11 VP7. Similar reactivity was observed when 2G4 was used to immunoprecipitate purified double-shelled virions. When soluble unassembled viral proteins were analyzed by ELISA, the 2G4 reactive pattern was as predicted from the parental origin of VP4. That is, 2G4 reacted with the soluble VP4 of reassortants having VP4 from SA11-Cl3 or SA11-4F and failed to react with VP4 of B223 origin, regardless of the origin of VP7. PRN and ELISA results obtained with nonglycosylated viruses revealed that the unexpected reactivity of 2G4 with virus particles was not the result of differential glycosylation of VP7 and epitope masking. These results indicate that the 2G4 epitope existed in the soluble form of VP4 encoded by SA11-Cl3 or SA11-4F but not in soluble B223 VP4. On the other hand, in assembled virions, the presentation of the 2G4 epitope on VP4 was unexpected in some reassortants and was affected by the specific interactions between VP4 and VP7 of heterologous parental origin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. W., Chen D., Estes M. K., Ramig R. F. Biological and immunological characterization of a simian rotavirus SA11 variant with an altered genome segment 4. Virology. 1989 Apr;169(2):427–435. doi: 10.1016/0042-6822(89)90168-2. [DOI] [PubMed] [Google Scholar]
- Burns J. W., Greenberg H. B., Shaw R. D., Estes M. K. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988 Jun;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caust J., Dyall-Smith M. L., Lazdins I., Holmes I. H. Glycosylation, an important modifier of rotavirus antigenicity. Arch Virol. 1987;96(3-4):123–134. doi: 10.1007/BF01320955. [DOI] [PubMed] [Google Scholar]
- Chen D., Burns J. W., Estes M. K., Ramig R. F. Phenotypes of rotavirus reassortants depend upon the recipient genetic background. Proc Natl Acad Sci U S A. 1989 May;86(10):3743–3747. doi: 10.1073/pnas.86.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., White J. R., Cotton R. G. Non-neutralizing monoclonal antibodies to a trypsin-sensitive site on the major glycoprotein of rotavirus which discriminate between virus serotypes. Arch Virol. 1987;93(3-4):199–211. doi: 10.1007/BF01310974. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Ramig R. F., Ericson B. L. Heterogeneity in the structural glycoprotein (VP7) of simian rotavirus SA11. Virology. 1982 Oct 15;122(1):8–14. doi: 10.1016/0042-6822(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Keith J., Nakagomi O., Nakagomi T., Askaa J., Kapikian A. Z., Chanock R. M., Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985 Mar;141(2):292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986 Jan;57(1):110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn P. R., Sato F., Powell K. F., Bellamy A. R., Napier J. R., Harding D. R., Hancock W. S., Siegman L. J., Both G. W. Rotavirus neutralizing protein VP7: antigenic determinants investigated by sequence analysis and peptide synthesis. J Virol. 1985 Jun;54(3):791–797. doi: 10.1128/jvi.54.3.791-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. Biochemical mapping of the simian rotavirus SA11 genome. J Virol. 1983 May;46(2):413–423. doi: 10.1128/jvi.46.2.413-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. G., Azeredo R. S., Fialho A. M., Vidal M. N. Genomic heterogeneity of simian rotavirus SA11. J Gen Virol. 1984 Apr;65(Pt 4):815–818. doi: 10.1099/0022-1317-65-4-815. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Galle K. L. Rotavirus genome segment 4 determines viral replication phenotype in cultured liver cells (HepG2). J Virol. 1990 Mar;64(3):1044–1049. doi: 10.1128/jvi.64.3.1044-1049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants of simian rotavirus SA11. Virology. 1982 Jul 15;120(1):93–105. doi: 10.1016/0042-6822(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Fong K. J., Losonsky G. A., Levine M. M., Maldonado Y., Yolken R., Flores J., Kapikian A. Z., Vo P. T., Greenberg H. B. Epitope-specific immune responses to rotavirus vaccination. Gastroenterology. 1987 Nov;93(5):941–950. doi: 10.1016/0016-5085(87)90555-5. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]