Abstract
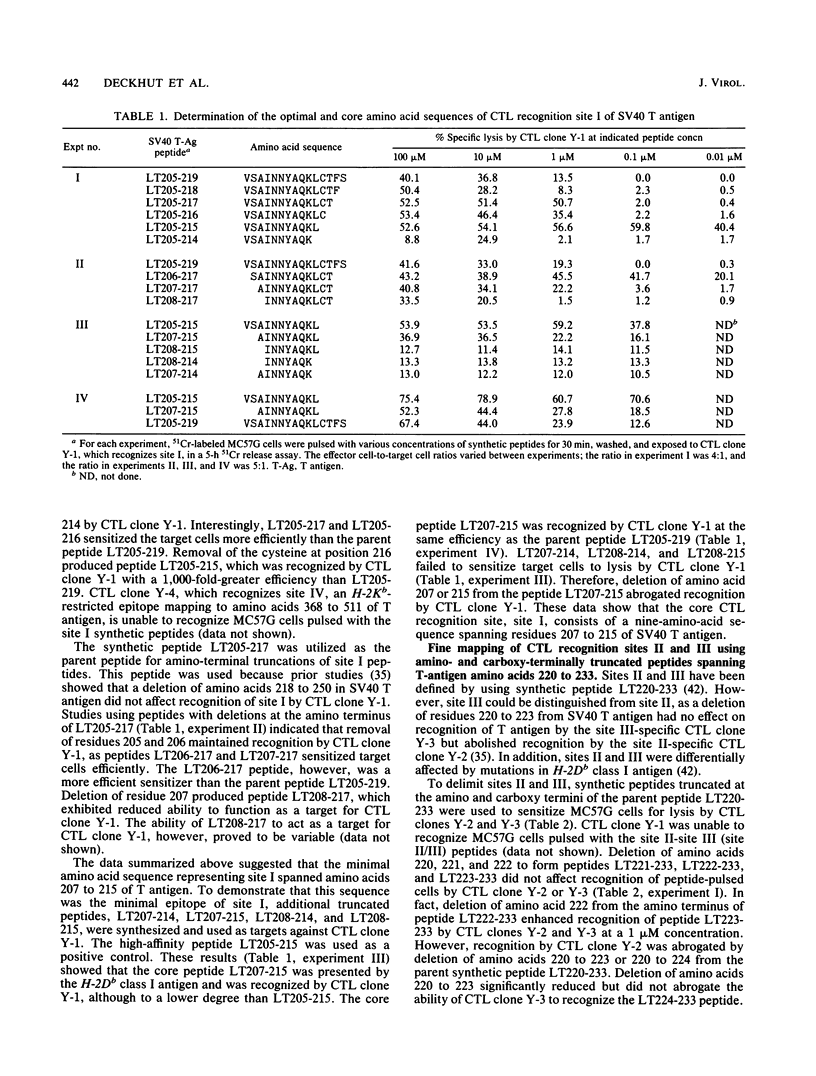
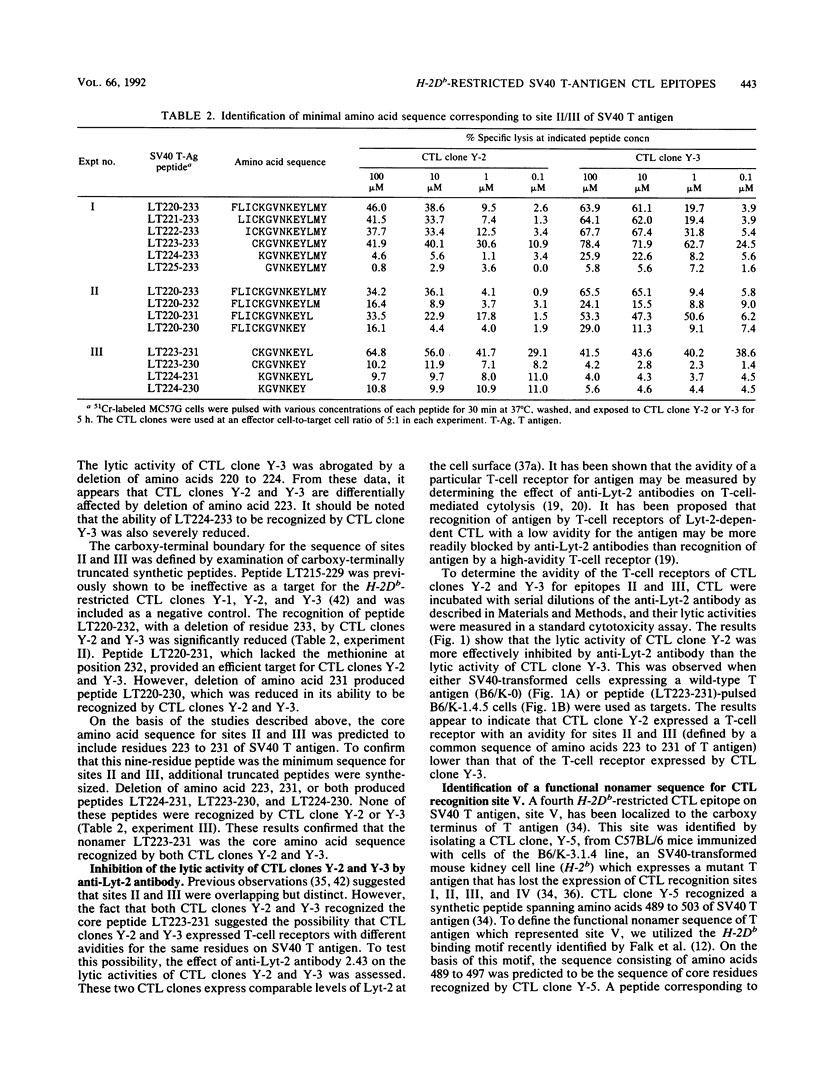
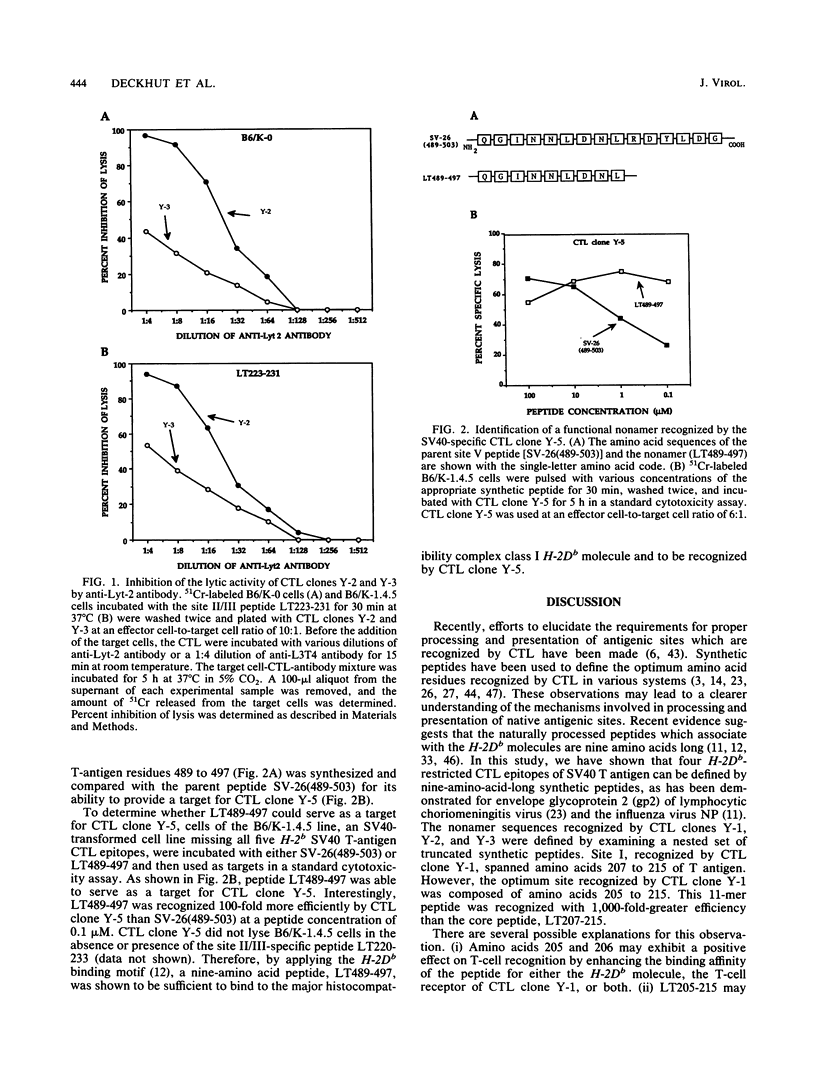
Simian virus 40 (SV40) tumor (T) antigen expressed in H-2b SV40-transformed cells induces the generation of Lyt-2+ (CD8+) cytotoxic T lymphocytes (CTL), which are involved in tumor rejection, in syngeneic mice. Five CTL recognition sites on T antigen have been described by using mutant T antigens. Four of the sites (I, II, III, and V) are H-2Db restricted and have been broadly mapped with synthetic peptides of 15 amino acids in length overlapping by 5 residues at the amino and carboxy termini. The goal of this study was to define the minimal and optimal amino acid sequences of T antigen which would serve as recognition elements for the H-2Db-restricted CTL clones Y-1, Y-2, Y-3, and Y-5, which recognizes sites I, II, III, and V, respectively. The minimal and optimal residues of T antigen recognized by the four CTL clones were determined by using synthetic peptides truncated at the amino or carboxy terminus and an H-2Db peptide-binding motif. The minimal site recognized by CTL clone Y-1 was defined as amino acids 207 to 215 of SV40 T antigen. However, the optimal sequence recognized by CTL clone Y-1 spanned T-antigen amino acids 205 to 215. The T-antigen peptide sequence LT223-231 was the optimal and minimal sequence recognized by both CTL clones Y-2 and Y-3. Site V was determined to be contained within amino acids 489 to 497 of T antigen. The lytic activities of CTL clones Y-2 and Y-3, which recognize a single nonamer peptide, LT223-231, were affected differently by anti-Lyt-2 antibody, suggesting that the T-cell receptors of these two CTL clones differ in their avidities. As the minimal and optimal H-2Db-restricted CTL recognition sites have been defined by nonamer synthetic peptides, it is now possible to search for naturally processed H-2Db-restricted epitopes of T antigen and identify critical residues involved in processing, presentation, and recognition by SV40-specific CTL.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. J., Lowen L. C., Mann D., Nishimura M., Hood L., Stroynowski I., Forman J. The Q7 alpha 3 domain alters T cell recognition of class I antigens. J Immunol. 1991 May 1;146(9):3082–3090. [PubMed] [Google Scholar]
- Anderson R. W., Tevethia M. J., Kalderon D., Smith A. E., Tevethia S. S. Fine mapping two distinct antigenic sites on simian virus 40 (SV40) T antigen reactive with SV40-specific cytotoxic T-cell clones by using SV40 deletion mutants. J Virol. 1988 Jan;62(1):285–296. doi: 10.1128/jvi.62.1.285-296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J., Rothbard J., Davey J., Jones I., Townsend A. Use of synthetic peptides of influenza nucleoprotein to define epitopes recognized by class I-restricted cytotoxic T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1508–1523. doi: 10.1084/jem.165.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataillon G., Pross H., Klein G. Comparative in vitro sensitivity of twom methylcholanthrene-induced murine sarcoma lines to humoral and cellular immune cytotoxicity. Int J Cancer. 1975 Aug 15;16(2):255–265. doi: 10.1002/ijc.2910160208. [DOI] [PubMed] [Google Scholar]
- Bates M. P., Jennings S. R., Tanaka Y., Tevethia M. J., Tevethia S. S. Recognition of simian virus 40 T antigen synthesized during viral lytic cycle in monkey kidney cells expressing mouse H-2Kb- and H-2Db-transfected genes by SV40-specific cytotoxic T lymphocytes leads to the abrogation of virus lytic cycle. Virology. 1988 Jan;162(1):197–205. doi: 10.1016/0042-6822(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Braciale V. L. Antigen presentation: structural themes and functional variations. Immunol Today. 1991 Apr;12(4):124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- Chang C., Martin R. G., Livingston D. M., Luborsky S. W., Hu C. P., Mora P. T. Relationship between T-antigen and tumor-specific transplantation antigen in simian virus 40-transformed cells. J Virol. 1979 Jan;29(1):69–75. doi: 10.1128/jvi.29.1.69-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Hansen T. H., Ingold A. L., Potter T. A. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I alpha 3 domain: CD8 and the T-cell receptor recognize the same class I molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Potter T. A., Wormstall E. M., Hansen T. H. The Lyt-2 molecule recognizes residues in the class I alpha 3 domain in allogeneic cytotoxic T cell responses. J Exp Med. 1988 Jul 1;168(1):325–341. doi: 10.1084/jem.168.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Deres K., Metzger J., Jung G., Rammensee H. G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991 Aug 1;174(2):425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Characterization of a progressive tumor from C3H fibroblasts transformed in vitro with SV40 virus. Immunoresistance in vivo correlates with phenotypic loss of H-2Kk. J Immunol. 1982 Sep;129(3):1306–1312. [PubMed] [Google Scholar]
- Gotch F., McMichael A., Rothbard J. Recognition of influenza A matrix protein by HLA-A2-restricted cytotoxic T lymphocytes. Use of analogues to orientate the matrix peptide in the HLA-A2 binding site. J Exp Med. 1988 Dec 1;168(6):2045–2057. doi: 10.1084/jem.168.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Fresa K. L., Lippe P. A., Milici J. E., Tevethia S. S. Frequency analysis of simian virus 40-specific cytotoxic T lymphocyte precursors in the high responder C57BL/6 mouse strain. J Gen Virol. 1988 Oct;69(Pt 10):2493–2503. doi: 10.1099/0022-1317-69-10-2493. [DOI] [PubMed] [Google Scholar]
- Joly E., Salvato M., Whitton J. L., Oldstone M. B. Polymorphism of cytotoxic T-lymphocyte clones that recognize a defined nine-amino-acid immunodominant domain of lymphocytic choriomeningitis virus glycoprotein. J Virol. 1989 May;63(5):1845–1851. doi: 10.1128/jvi.63.5.1845-1851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Thiernesse N., Cerottini J. C. Inhibition of T cell-mediated cytolysis by monoclonal antibodies directed against Lyt-2: heterogeneity of inhibition at the clonal level. J Immunol. 1981 May;126(5):1671–1675. [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Cerottini J. C., Corradin G. Synthetic peptides as antigens and competitors in recognition by H-2-restricted cytolytic T cells specific for HLA. J Exp Med. 1988 Apr 1;167(4):1391–1405. doi: 10.1084/jem.167.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. N., Morrison L. A., Gorka J., Braciale V. L., Braciale T. J. The recognition of a viral antigenic moiety by class I MHC-restricted cytolytic T lymphocytes is limited by the availability of the endogenously processed antigen. J Immunol. 1990 Nov 15;145(10):3188–3193. [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Rajan T. V., Dick R. F., 2nd, Bluestone J. A. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989 Jan 5;337(6202):73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- Pretell J., Greenfield R. S., Tevethia S. S. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). V In vitro demonstration of SV40 TrAg in SV40 infected nonpermissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979 Aug;97(1):32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Rawle F. C., O'Connell K. A., Geib R. W., Roberts B., Gooding L. R. Fine mapping of an H-2Kk restricted cytotoxic T lymphocyte epitope in SV40 T antigen by using in-frame deletion mutants and a synthetic peptide. J Immunol. 1988 Oct 15;141(8):2734–2739. [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Rosenstein Y., Ratnofsky S., Burakoff S. J., Herrmann S. H. Direct evidence for binding of CD8 to HLA class I antigens. J Exp Med. 1989 Jan 1;169(1):149–160. doi: 10.1084/jem.169.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Schumacher T. N., De Bruijn M. L., Vernie L. N., Kast W. M., Melief C. J., Neefjes J. J., Ploegh H. L. Peptide selection by MHC class I molecules. Nature. 1991 Apr 25;350(6320):703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Anderson R. W., Maloy W. L., Tevethia S. S. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology. 1989 Jul;171(1):205–213. doi: 10.1016/0042-6822(89)90527-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia M. J., Kalderon D., Smith A. E., Tevethia S. S. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology. 1988 Feb;162(2):427–436. doi: 10.1016/0042-6822(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia S. S. In vitro selection of SV40 T antigen epitope loss variants by site-specific cytotoxic T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4348–4354. [PubMed] [Google Scholar]
- Tanaka Y., Tevethia S. S. Loss of immunorecessive cytotoxic T lymphocyte determinant V on SV40 T antigen following cocultivation with site-specific cytotoxic T lymphocyte clone Y-5. Intervirology. 1990;31(2-4):197–202. doi: 10.1159/000150154. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Flyer D. C., Tjian R. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). VI. Mechanism of induction of SV40 transplantation immunity in mice by purified SV40 T antigen (D2 protein). Virology. 1980 Nov;107(1):13–23. doi: 10.1016/0042-6822(80)90268-8. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Greenfield R. S., Flyer D. C., Tevethia M. J. SV40 transplantation antigen: relationship to SV40-specific proteins. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):235–242. doi: 10.1101/sqb.1980.044.01.027. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Lewis A. J., Campbell A. E., Tevethia M. J., Rigby P. W. Simian virus 40 specific cytotoxic lymphocyte clones localize two distinct TSTA sites on cells synthesizing a 48 kD SV40 T antigen. Virology. 1984 Mar;133(2):443–447. doi: 10.1016/0042-6822(84)90411-2. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Lewis M., Tanaka Y., Milici J., Knowles B., Maloy W. L., Anderson R. Dissection of H-2Db-restricted cytotoxic T-lymphocyte epitopes on simian virus 40 T antigen by the use of synthetic peptides and H-2Dbm mutants. J Virol. 1990 Mar;64(3):1192–1200. doi: 10.1128/jvi.64.3.1192-1200.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990 Feb;7(1):83–96. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Berzofsky J. A., Krzych U., Smith J. A., Hodes R. J., Finnegan A. Sequences outside a minimal immunodominant site exert negative effects on recognition by staphylococcal nuclease-specific T cell clones. J Immunol. 1989 Nov 1;143(9):2814–2819. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Gebhard J. R., Lewicki H., Tishon A., Oldstone M. B. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988 Mar;62(3):687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



