Abstract
Bright objects capture our attention by virtue of ‘popping out’ from their surroundings. This correlates with strong responses in cortical areas thought to be important in attentional allocation. Previous studies have suggested that with the right mindset or training, humans can ignore popout stimuli. We studied the activity of neurons in monkey lateral intraparietal area while monkeys performed a visual search task. The monkeys were free to move their eyes, and a distractor, but never the search target, popped out. On trials in which the monkeys made a saccade directly to the search target, the popout distractor evoked a smaller response than the non-popout distractors. The intensity of the response to the popout correlated inversely with the monkeys’ ability to ignore it. We suggest that this modulation corresponds to a top-down mechanism that the brain uses to adjust the parietal representation of salience.
Visual search tasks have been commonly used by psychologists to investigate how subjects select a relevant object from a background of irrelevant ones. Many theories of attention assert that this selection occurs by an interaction between ‘bottom-up’ and ‘top-down’ control1–3. Bottom-up control refers to stimulus-driven processing that occurs in a parallel manner across the entire visual field. It is based exclusively on the properties of the stimulus and is unaffected by any advance knowledge about the stimulus or by any behavioral strategy on the part of the subject. It is responsible for the automatic capture of attention by salient objects that stand out from the background. Objects can also be selected because they match the viewer’s expectations. In this case, attention is guided through a top-down, or goal-directed, process that selects the stimuli on the basis of the viewer’s advance knowledge, intentions and goals. Studies that have investigated how bottom-up and top-down mechanisms compete for the control of attention have generally found that in the presence of a salient stimulus, the bottom-up activation prevails and attention is oriented automatically and involuntarily to a stimulus that stands out from the background, even when it is irrelevant for the task4–9. However, other studies have challenged this idea claiming that, with extended practice, attention and gaze are not always driven automatically and involuntarily to a salient, but irrelevant, stimulus when subjects know the properties of the target in advance and when these properties do not overlap with those of the distractor10–19.
Here we addressed the issue of whether monkeys are able to ignore a predictably irrelevant stimulus that pops out by virtue of its physical properties, and whether the behavior is reflected in the neural activity in posterior parietal cortex.
Models of target selection or attentional allocation often include a map of visual space, termed a salience map, which is used to identify an appropriate target by taking into account information from the scene and from cognitive influences20,21. This map is a two-dimensional topographic map in which neural activity represents the salience of objects within the visual environment. In this context, stimuli can be considered salient because of their physical properties, their behavioral relevance or both. Attention is then directed, in a winner-take-all manner, to the location that corresponds to the object that receives the highest activation22,23.
Visual salience is represented in a number of brain areas: the frontal eye fields24, area 7a (refs. 25–27), the lateral intraparietal area22,28,29 and the superior colliculus30. A singleton popout stimulus is represented in these areas by stronger responses than those to the same stimulus when it is not unique, whether it is behaviorally relevant24,26,31,32 or not27,33. In this study we examined the responses of neurons from the lateral intraparietal area (LIP) to see how the enhanced bottom-up response of a popout stimulus competes with the top-down knowledge that the popout stimulus is an irrelevant distraction.
RESULTS
Behavior
Two adult male rhesus monkeys were trained to perform a visual search task. The monkeys were free to move their eyes and reported their decision by a hand movement34. Each monkey initiated a trial by fixating a small spot in the middle of the screen, which appeared after it had grasped the two bars in its chair. After 1–1.75 s, the fixation point was extinguished and an array of eight crosses appeared (Fig. 1). One cross (the target) was an inverted or upright T and the monkey was rewarded for correctly indicating its orientation by releasing the appropriate bar. During this time the monkey was completely free to move its eyes—the trial did not end until a bar was released or a 3-s time limit was reached. Of the remaining seven distractors, six had the same luminance, color, width and height as the target, but differed only in the position at which the horizontal line crossed the vertical line. The remaining distractor was the same shape as the other distractors, but popped out by virtue of its bright luminance and green color. Monkeys correctly performed the task in about 95% of the trials, and even though they did not have to make a saccade to the target, they usually fixated it before releasing the bar. In a small percentage of trials (1–12%, depending on the day), monkey Z gave the correct manual response but never actually fixated the target.
Figure 1.
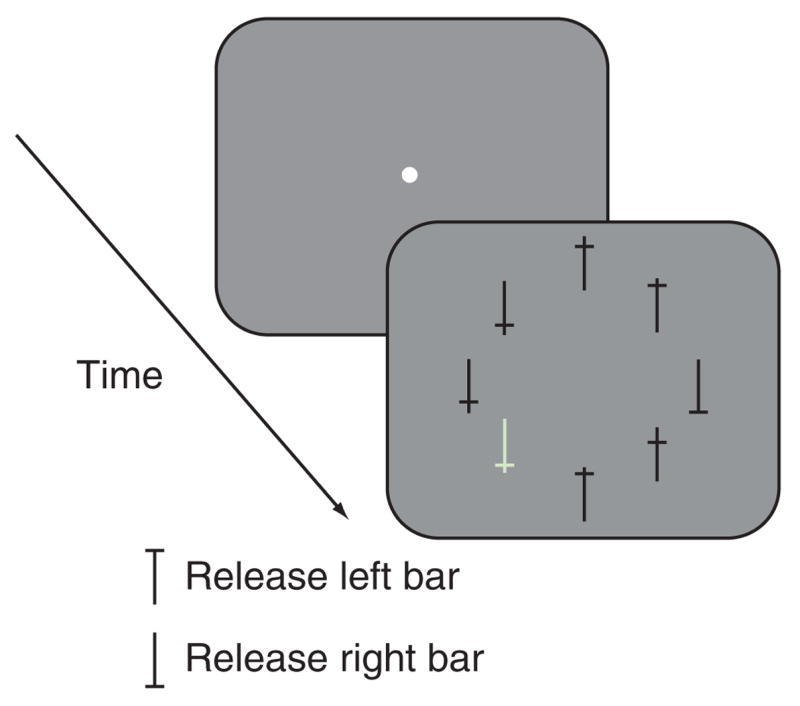
Behavioral task. The monkeys began the task by grabbing two bars attached to the primate chair. A fixation point appeared, which the monkey had to look at for 1–1.75 s, after which it disappeared and was replaced by an eight-stimulus array positioned along an imaginary circle such that one member of the array was in the center of the receptive field of the neuron under study. One of the stimuli was the target and the remaining seven were distractors. In every trial, one of the distractors (a bright green stimulus) popped out. The monkeys had 3 s to report the orientation of the target by releasing one of the two bars. No constraints were imposed on the monkeys’ eye movements; they were not required to fixate the target before giving the response and were not penalized for not fixating the target.
The monkeys’ response to the popout changed with experience. Initially, because we wanted to demonstrate the similarity between the monkey’s search strategy and that of humans, we often made the popout stimulus serve as the target of the search (A.E. Ipata, B.S. Krishna, J.W. Bisley, J. Gottlieb & M.E. Goldberg. Vis. Sci. Soc. Abstr. 3, 622a, 2003). In one variation of this experiment, we alternated blocks of trials in which the popout was always the target with blocks in which the popout was never the target. In blocks in which the popout was the target, the monkeys made their first saccade to the popout in almost all of the trials. In blocks in which the popout was never the target (Fig. 2, left columns), monkey R made the first saccade to the popout in 148 of 919 trials (16%), to the target in 520 of 919 trials (57%) and to a non-popout distractor in 251 of 919 trials (27%). Monkey Z made the first saccade to the popout in 517 of 2,438 trials (21%), to the target in 920 of 2,438 trials (38%) and to a non-popout distractor in 1,001 of 2,438 trials (41%).
Figure 2.
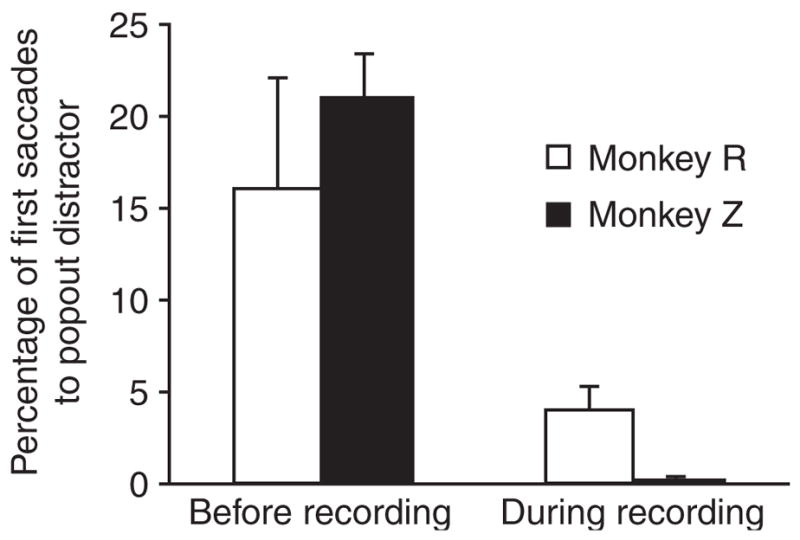
Percentage of first saccades to the popout distractor before and during recording. In sessions before recording, blocks in which the popout was the target were occasionally presented. Left, data from these sessions, but only from blocks in which the popout was always a distractor. Right, data from recording sessions, in which the popout was never the target. Error bars show s.e.m.
During the recording experiments, we were interested in trials in which the monkey made saccades away from the target, so we never made the target the popout. Under these circumstances (Fig. 2, right columns), the percentage of first saccades to the popout dropped to 4.0% in monkey R (1,612 of 39,902) and to 0.5% in monkey Z (150 of 31,923). Monkey R made 22,427 of 39,902 (56%) first saccades to the target and 15,863 of 39,902 (40%) first saccades to non-popout distractors; monkey Z made 14,066 of 31,923 (44%) first saccades to the target and 17,769 of 31,923 (56%) first saccades to non-popout distractors.
Neural activity
We collected data from 73 LIP neurons from two monkeys (42 in monkey R, 31 in monkey Z). We mapped a neuron’s receptive field using a memory-guided saccade task35. All neurons examined in this study had visually evoked activity when the target of the memory-guided saccade appeared in the neuron’s receptive field, and 78% of them responded significantly during the delay period (P < 0.05; t-test). For each neuron, we positioned the eight-stimulus array of the search task so that one stimulus was always in the center of the neuron’s receptive field. Under conditions of visual search, neurons in LIP accurately predict the direction and latency of the impending saccade34. To insure that the activity we studied was not contaminated by the saccadic signal, we only included in our analysis one-saccade trials, in which the monkey made a saccade to the target and released the appropriate bar (56% and 44% of all trials in monkeys R and Z, respectively). Because the target was in the center of the neuron’s receptive field on only about 12.5% of trials, the analysis included three different trial types: trials in which the monkey made a saccade to the target in the receptive field; trials in which the monkey made a saccade to the target, and a non-popout distractor was in the receptive field; and trials in which the monkey made a saccade to the target, and the popout distractor was in the receptive field. In all the neural data presented here, the target was never the popout.
We obtained spike density functions for single neurons (Fig. 3a,b) by sorting the data into trials in which the target (dark gray traces), the non-popout distractors (black traces) or the bright green popout distractor (light gray traces) were in the neuron’s receptive field. Note that the trials are averaged over left- and right-handed manual responses, and the black and light gray traces are averaged over all saccade directions except for that toward the receptive field. We aligned the data by the onset of the stimulus array (Fig. 3a) and by the onset of the initial targeting saccade (Fig. 3b). It was clear that the response to the popout distractor was less than the response to the non-popout distractor, and that both were substantially less than the response to the target to which the monkey was making the saccade. Note that the neuronal activity distinguished between popout and non-popout distractors about 90 ms after the onset of the search array.
Figure 3.
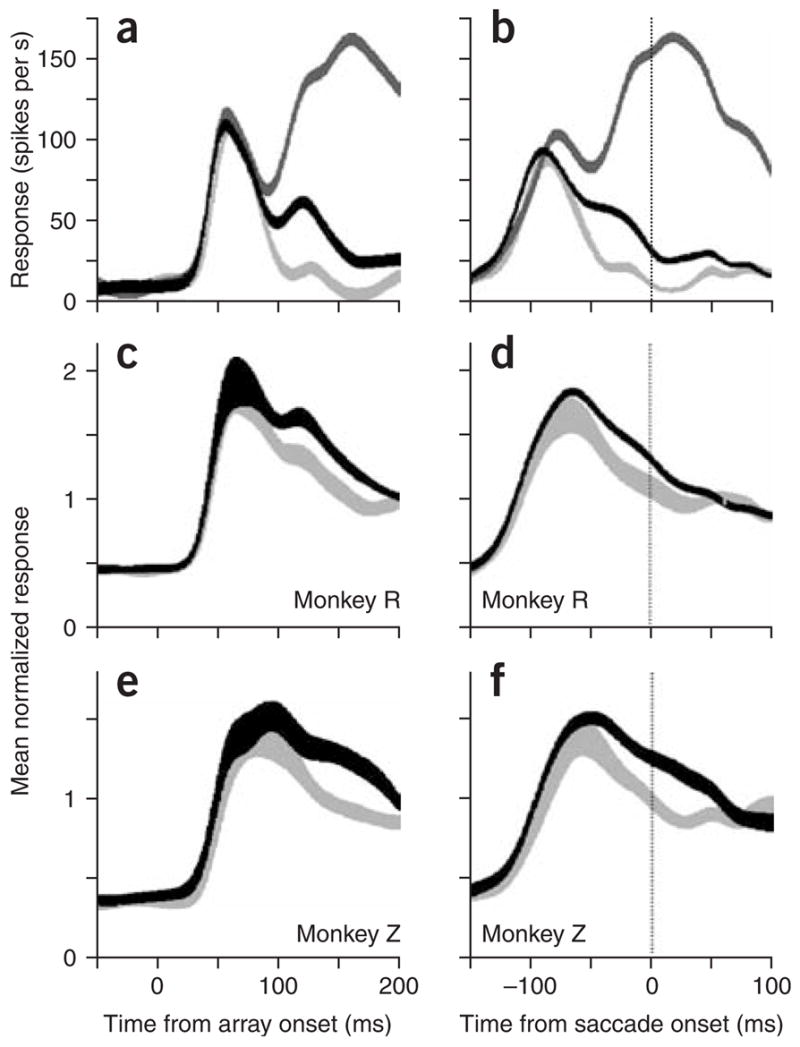
Responses to the target or distractors within the neuron’s receptive field. All data are from trials in which the monkey made the first saccade to the target and released the correct bar. (a) Responses of a single cell to the appearance of an array object in the receptive field, versus time from target onset. Dark gray trace, response to the target in the receptive field when the monkey made a saccade to the target. Black trace, response to a non-popout distractor in the receptive field when the monkey made a saccade to the target (located elsewhere). Light gray trace, response to the popout distractor in the receptive field when the monkey made a saccade to the target (located elsewhere). (b) Same data as in a, but aligned on saccade onset. (c,d) Population averages for monkey R aligned to array onset (c) or saccade onset (d). (e,f) As in c and d, but for monkey Z. Thickness of the traces shows s.e.m.
This differentiation between popout and non-popout distractor was present across the sample of neurons, both in the population averages and in the activity of the single neurons. The mean spike density histograms for monkeys R and Z, calculated from all cells in which there were at least five trials of each type (41 cells in Monkey R, 29 in Monkey Z) are shown aligned on array onset and on saccade onset for monkey R (Fig. 3c,d) and for monkey Z (Fig. 3e,f). As in the single-cell example, the responses to the two classes of distractor were initially similar, but diverged to give a consistently weaker response to the popout distractor.
The latency of the response to the popout distractor, calculated using the method described previously36, was slightly longer than that to the non-popout distractor for almost all the cells (mean ± s.e.m. of the difference across the cells: 9.1 ± 1.6 and 8.9 ± 2.2 for monkeys R and Z, respectively; Wilcoxon sign-rank tests: P < 0.0004). Therefore, we compared the activity in each epoch starting from the latency of response of each cell in each condition (popout versus non-popout distractor), rather than from the onset of the search array. This meant that we analyzed the data from the 48 neurons for which latencies could be calculated. As in the pooled population response, there was little difference between the popout and non-popout distractor conditions in the first 40 ms after the latency of the initial responses (P = 0.88, monkey R; P = 0.43, monkey Z; Wilcoxon sign-rank tests; Fig. 4a,b). In the following 50-ms epoch (Fig. 4c,d), the average responses were already significantly less for the popout distractor than for the non-popout distractor (P = 0.0008, monkey R; P = 0.004, monkey Z). Similar results were also seen when the data from all neurons were analyzed with a 50-ms epoch centered on saccade onset (Fig. 4e,f; P = 0.005, monkey R; P = 0.001, monkey Z).
Figure 4.
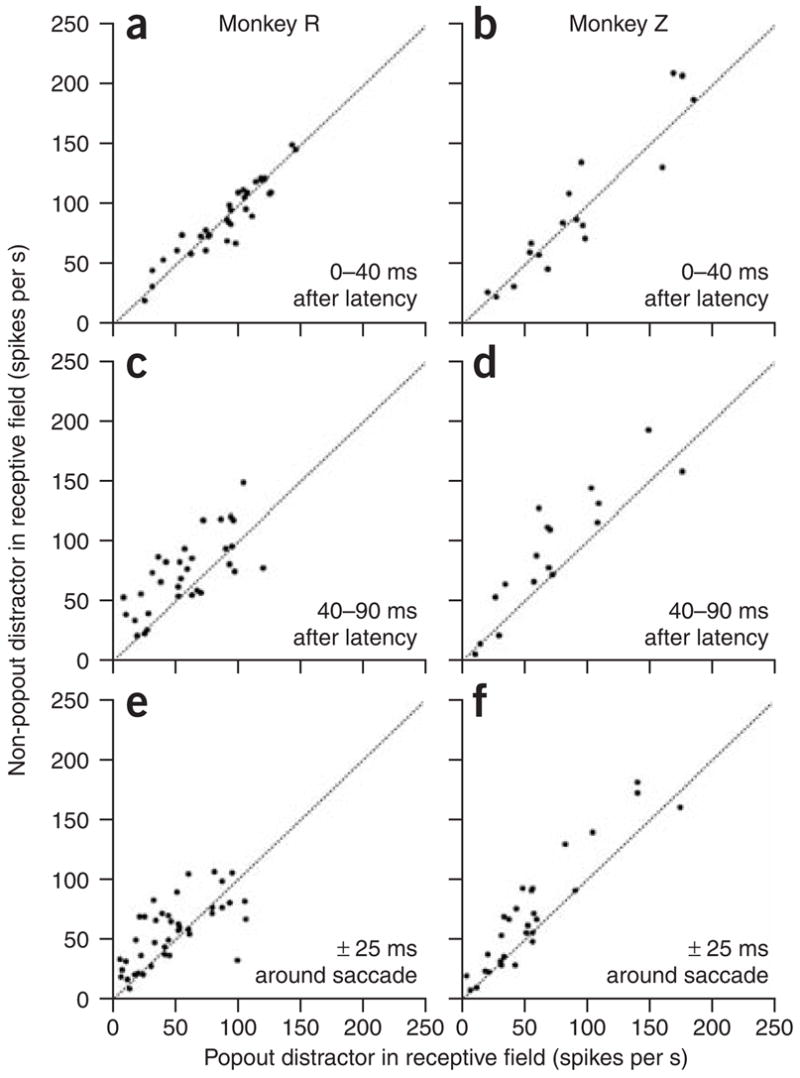
Response to popout and non-popout distractors in three epochs of the trial for each monkey. (a,b) The response of each cell to the non-popout distractor is plotted against the response of the same cell to the popout distractor in the first 40 ms after the latency. (c,d) The responses in a 50-ms epoch after the first response, plotted as in a. (e,f) The responses in the interval ± 25 ms around saccade onset, plotted as in a.
On days when the monkeys were unable to suppress saccades to the popout distractor, neuronal responses to the popout distractor were equal to, or stronger than, the responses to the non-popout distractors. For each cell recorded, we calculated the proportion of first saccades made to the popout distractor within that block of trials, and used this as a metric that showed how well the monkey was able to overtly ignore the popout distractor. We then plotted these data against the difference between the responses to the non-popout and popout distractors for the cell recorded in that block, in a 50-ms epoch starting at 80 ms after array onset (Fig. 5). For monkey R, it was clear that whenever the differences were positive (that is, the response to the popout was weaker), the monkey generally was able to inhibit saccades to the popout stimulus (Fig. 5a). However, in sessions in which the monkey was unable to suppress saccades toward the popout (that is, those in which more than 5% of trials began with the monkey making a saccade to the popout), the responses to the popout stimulus were either equal to or greater than the responses to the non-popout stimuli. We used linear regression to fit the data and found that there was a significant correlation between the ability of the monkey to ignore the popout and the difference in activity (P = 0.001). The results from monkey Z were less obvious (Fig. 5b), as this monkey almost always suppressed saccades to the popout. However, in line with this behavior, monkey Z had only three sessions in which the neuron being studied clearly responded more to the popout distractor than to the non-popout distractors; moreover, in the one session in which he was unable to suppress saccades to the popout, the response to the popout stimulus was not significantly lower than the response to the non-popout stimulus (P = 0.75).
Figure 5.
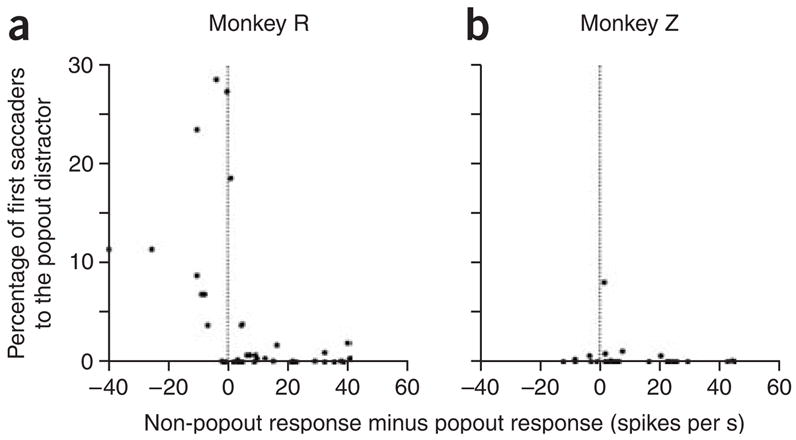
Cell-by-cell correlation of response suppression with saccade suppression. (a,b) Percentage of trials in which the first saccade was made to the popout distractor for each cell, versus the difference in the number of spikes between the responses (within the time epoch from 80 ms to 130 ms after array onset) to the non-popout and popout distractors for the cell recorded in that session.
DISCUSSION
In this study, monkeys learned to perform a free-viewing visual search task in which the most conspicuous object was not the target, but one of the distractors. We have demonstrated that with training, monkeys were able to ignore this popout stimulus overtly. We found that the responses of neurons in LIP to the popout stimuli were always slower and usually weaker than the responses to the other distractors. Furthermore, these responses were at their weakest on days when the monkeys were most successfully able to ignore the popout. On days when the monkeys were least able to ignore the popout, the responses to the popout were often stronger than the responses to the non-popout distractors. Because the performance remained constant within a single recording session, we suggest that the reduction of activity represents ongoing day-to-day top-down control that suppresses the importance of the popout on the parietal salience map29, thereby facilitating a more rapid discovery of the target.
The finding that monkeys were able to ignore an irrelevant popout stimulus is consistent with results from human studies, which have shown that stimulus-driven capture can be over-ridden by goal-directed selection of a target stimulus that has features that separate it from the popout distractor13,14,18. Recent findings have suggested that such resistance to stimulus-driven capture might be mediated by top-down inhibition16.
Previous physiological studies have found that the responses in posterior parietal cortex to singleton popout stimuli are greater than those elicited by the same stimuli when they are not popouts26, even when they are irrelevant27. This is supported by preliminary data from early training in monkey R: during this time, the task was evolving, and thus a strategy of continually ignoring the popout would have been detrimental. These data showed that the average response to the popout distractor was higher than that to the non-popout distractor (P = 0.04, n = 10; Wilcoxon sign-rank test). When monkeys were exclusively given the task in which the popout stimulus was always the distractor, we saw a lower response to the popout; we argue that this reflects the effect of top-down control and that the strength of this reduction depends on the attentional set adopted by the monkey. The reduced response to the popout was manifested as a later decrement of response, after a brief period in which the response to the popout was not different from the response to the non-popout distractor. This, coupled with the daily fluctuation of the popout response, suggests that the difference in response is not the result of experience-dependent plasticity37, but, instead, of volitional, attentional modulation. On days when the monkeys were less able to ignore the popout, there was an absence or a reversal of the difference in activity; this is consistent with previous reports that illustrate the normal enhancing effect of bottom-up salience in posterior parietal cortex26,27.
The slower latency in response to the popout distractor could be attributed either to a color-selective trait of the neurons or to a long-term influence such as perceptual learning. Typically, LIP neurons are not color-selective, unless the color is relevant for behavior38, so we think that this is an unlikely explanation for the latency difference. Furthermore, although we did not record enough activity to calculate latencies from the ten LIP neurons recorded during initial training, the results from area 7a (ref. 27) suggest that although stimuli evoke stronger parietal responses when they popout than when they are identical to others in an array, the responses do not exhibit latency differences. The fact that the shift of neuronal latency occurs at the beginning of the neuronal response suggests that it may be introduced at an earlier level of the visual system. It is well known that extended training modifies basic neuronal response properties at both early37 and intermediate39 levels of visual cortex. Thus, we argue that in addition to the top-down influences, there is a bottom-up learning effect that changes the connections between the ascending inputs from early visual areas and LIP, and that this manifests itself in a prolonged latency.
Models of attention and saccade target selection have hypothesized the presence of a two-dimensional topographic salience map in the brain controlling the deployment of attention and saccadic eye movements1,20,21,40. The activity of this map represents a combination of bottom-up physical salience and top-down influences that render inconspicuous objects behaviorally salient. LIP has been proposed as one of the brain areas that provide such a map28,29,41. As such, it is worth remembering that all the neural data shown here come from trials in which the monkey, even if not forced to ignore the popout, did not make a saccade to the popout stimulus but went straight to the target. Thus, our data show that top-down and bottom-up factors interact and influence the entire salience map in LIP, as all the differences in activity that we have shown are not at the spatial location of the monkey’s area of overt attention—that is, around the target. Although there have been previous attempts to examine the interactions between top-down and bottom-up influences in the brain24,42, these have been done at a region of the map to which a saccade was to be made and thus the responses were certainly influenced by the motor plan itself. Previous studies have shown that in a free-viewing visual search task34, neuronal activity in LIP reliably predicts when and in which direction a saccade will occur, although it does not do so during the delay period of a delayed saccade task22,43. However the activity during the delay can be used to predict where attention will be allocated22,23,43. A lower response in LIP therefore makes the stimulus evoking the response less likely to be the target of a saccade (which we have demonstrated in these experiments) and, we suggest, less likely to be attended (which we have not demonstrated here). So, although there may still be some debate in the human psychophysics literature about whether we can truly ignore a singleton stimulus44, the present data provide strong evidence that it is possible in the monkey, in terms of both saccade target selection and attentional allocation.
METHODS
Two male rhesus monkeys (Macaca mulatta) weighing 8–14 kg were used in this experiment. All experimental protocols were approved by the Animal Care and Use Committees at Columbia University and the New York State Psychiatric Institute, and complied with the guidelines established by the Public Health Service Guide for the Care and Use of Laboratory Animals.
Monkeys had subconjunctival scleral search coils45, head-restraining devices and recording chambers implanted during aseptic surgery under ketamine and isoflurane anesthesia. Chambers were positioned over the intraparietal sulcus, guided by magnetic resonance images.
Behavioral procedure
Monkeys were trained on a free-viewing visual search task. They sat in a dimly illuminated room with their head fixed and viewed a tangent screen that stood approximately 75 cm away. The sequence of a single trial is shown in Figure 1. Each trial started when the monkey grabbed one of two bars with each hand, then a small white central fixation point appeared in the center of a gray background. If the monkey maintained its fixation inside a 3° × 3° window for 1–1.75 s, the fixation point disappeared and an array of eight stimuli appeared. The array consisted of a target and seven distractors that were positioned around an imaginary circle, equally spaced around the former fixation spot, such that one stimulus was always in the center of the neuron’s receptive field. On each trial the relative position of all the stimuli changed randomly. The target was a black capital T that could be upright or inverted. The orientation of the target on each trial was unpredictable. Six of the distractors had the same dimensions, color and luminance as the target, but differed only in the position at which the horizontal component crossed the vertical component. One of the distractors, designated the popout distractor, had the same dimensions as the other distractors but popped out by virtue of its color and luminance (bright green). Monkeys were rewarded for reporting the orientation of the target by releasing one of the two bars (the left bar when the target was the upright capital T and the right bar when the target was the inverted capital T). Monkeys were free to move their gaze and did not need to fixate the target to receive the reward. They were given 3 s to respond correctly, after which the trial aborted. Initially, monkeys were trained and then extensively tested on a variation of this task in which three blocks were interleaved: one in which the task was identical to that in the present study (that is, a distractor always popped out); one in which there was no popout stimulus; and one in which the target always popped out.
Recording
Single units were recorded from area LIP with glass-insulated tungsten electrodes (Alpha Omega Engineering). The electrodes were introduced through a guide tube positioned in a 1 mm × 1 mm spaced grid. Action potentials were amplified, filtered and discriminated using an amplitude window discriminator.
Once a neuron was isolated, the receptive field was mapped by positioning a white spot in different locations in the visual field while monkeys performed a memory-guided saccade task35. We studied a total of 73 neurons in LIP (42 from monkey R; 31 from monkey Z) while the monkeys performed the visual search task. Every neuron we studied responded significantly to the target of the memory-guided saccade, and 78% of neurons in monkey R and 80% in monkey Z responded significantly during the delay (P < 0.05, t-test).
After mapping the receptive field, we recorded from the same neuron while the monkey performed the free-viewing visual search task (see above). In this experiment, we analyzed the activity of the neurons during the interval between the onset of the array and 25 ms after the onset of the first saccade, and restricted our analyses to only those trials in which the monkey made one saccade directly to the target.
All data analysis programs were written in Matlab (Mathworks). To examine the pattern of activity, we calculated spike-density functions by convolving the spike train, sampled at 1 kHz, with a Gaussian of σ 10 ms (ref. 46). The neuronal response is illustrated as the average of this spike-density trace over the interval of interest. To create the population data, we took the square root of every data point from all the spike-density functions for that cell. We then divided each value by the total mean of the square-rooted values from all the functions. We used this square-root normalization method to decrease the effect of outliers47. More detailed analyses were done on actual spike rates taken from 30- or 50-ms epochs.
Response latencies were calculated using the Poisson fit threshold method48,49 described in detail previously36. Briefly, we derived a Poisson distribution from the baseline activity 100 ms before array onset, analyzed in 2-ms bins. We then set the threshold as the 99th percentile of the Poisson fit. Finally, we defined the latency as the first of three consecutive 2-ms bins, each of which contained more spikes than the threshold.
Acknowledgments
We thank M. Osman and G. Asfaw for veterinary care, Y. Pavlova for expert assistance with animal care, G. Duncan for electronic and systems work and L. Palmer for her indispensable help. Preliminary experiments were performed at the Laboratory of Sensorimotor Research, National Eye Institute, Bethesda, Maryland. The research was supported by grants to M.E.G. from the National Eye Institute (R01 EY014978-01 and R24 EY015634-01), the Whitehall, James S. MacDonnell and W.M. Keck Foundations, and the David Mahoney Chair at Columbia University; and to A.L.G. from the National Science Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
A.E.I. and A.L.G. performed the experiments, analyzed the data, prepared the figures and contributed to the manuscript preparation. J.G. first suggested studying the response to a task-irrelevant popout in a search task. J.W.B. and M.E.G. supervised the experiments, the analysis of the data and the preparation of the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Published online at http://www.nature.com/natureneuroscience
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Cave KR, Wolfe JM. Modeling the role of parallel processing in visual search. Cognit Psychol. 1990;22:225–271. doi: 10.1016/0010-0285(90)90017-x. [DOI] [PubMed] [Google Scholar]
- 2.Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychol Rev. 1989;96:433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- 3.Treisman AM, Gelade G. A feature-integration theory of attention. Cognit Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- 4.Joseph JS, Optican LM. Involuntary attentional shifts due to orientation differences. Percept Psychophys. 1996;58:651–665. doi: 10.3758/bf03213098. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DE, Colcombe AM, Kramer AF, Hahn S. Attentional and oculomotor capture by onset, luminance and color singletons. Vision Res. 2000;40:1443–1458. doi: 10.1016/s0042-6989(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 6.Theeuwes J. Cross-dimensional perceptual selectivity. Percept Psychophys. 1991;50:184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- 7.Yantis S, Hillstrom AP. Stimulus-driven attentional capture: evidence from equiluminant visual objects. J Exp Psychol Hum Percept Perform. 1994;20:95–107. doi: 10.1037//0096-1523.20.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Theeuwes J, Burger R. Attentional control during visual search: the effect of irrelevant singletons. J Exp Psychol Hum Percept Perform. 1998;24:1342–1353. doi: 10.1037//0096-1523.24.5.1342. [DOI] [PubMed] [Google Scholar]
- 9.Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- 10.Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- 11.Kim MS, Cave KR. Top-down and bottom-up attentional control: on the nature of interference from a salient distractor. Percept Psychophys. 1999;61:1009–1023. doi: 10.3758/bf03207609. [DOI] [PubMed] [Google Scholar]
- 12.Theeuwes J, De Vries GJ, Godijn R. Attentional and oculomotor capture with static singletons. Percept Psychophys. 2003;65:735–746. doi: 10.3758/bf03194810. [DOI] [PubMed] [Google Scholar]
- 13.Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- 14.Yantis S, Egeth HE. On the distinction between visual salience and stimulus-driven attentional capture. J Exp Psychol Hum Percept Perform. 1999;25:661–676. doi: 10.1037//0096-1523.25.3.661. [DOI] [PubMed] [Google Scholar]
- 15.Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: evidence for a competitive integration model. J Exp Psychol Hum Percept Perform. 2002;28:1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- 16.Lamy D, Tsal Y, Egeth HE. Does a salient distractor capture attention early in processing? Psychon Bull Rev. 2003;10:621–629. doi: 10.3758/bf03196524. [DOI] [PubMed] [Google Scholar]
- 17.Lamy D, Leber A, Egeth HE. Effects of task relevance and stimulus-driven salience in feature-search mode. J Exp Psychol Hum Percept Perform. 2004;30:1019–1031. doi: 10.1037/0096-1523.30.6.1019. [DOI] [PubMed] [Google Scholar]
- 18.Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- 19.Rauschenberger R. Attentional capture by auto- and allo-cues. Psychon Bull Rev. 2003;10:814–842. doi: 10.3758/bf03196545. [DOI] [PubMed] [Google Scholar]
- 20.Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219–227. [PubMed] [Google Scholar]
- 21.Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res. 2000;40:1489–1506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 22.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 23.Bisley JW, Goldberg ME. Neural correlates of attention and distractibility in the lateral intraparietal area. J Neurophysiol. 2006;95:1696–1717. doi: 10.1152/jn.00848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz MA, Constantinidis C. Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb Cortex. 1995;5:448–456. doi: 10.1093/cercor/5.5.448. [DOI] [PubMed] [Google Scholar]
- 26.Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple-stimulus displays: I. Neurons encode the location of the salient stimulus. Cereb Cortex. 2001;11:581–591. doi: 10.1093/cercor/11.7.581. [DOI] [PubMed] [Google Scholar]
- 27.Constantinidis C, Steinmetz MA. Posterior parietal cortex automatically encodes the location of salient stimuli. J Neurosci. 2005;25:233–238. doi: 10.1523/JNEUROSCI.3379-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusunoki M, Gottlieb J, Goldberg ME. The lateral intraparietal area as a salience map: the representation of abrupt onset, stimulus motion, and task relevance. Vision Res. 2000;40:1459–1468. doi: 10.1016/s0042-6989(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- 30.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- 31.Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- 32.Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- 33.Bichot NP, Rao SC, Schall JD. Continuous processing in macaque frontal cortex during visual search. Neuropsychologia. 2001;39:972–982. doi: 10.1016/s0028-3932(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 34.Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 36.Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth LJ, Assad JA. Dynamic coding of behaviourally relevant stimuli in parietal cortex. Nature. 2002;415:165–168. doi: 10.1038/415165a. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfe JM. Guided search 2.0. A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- 41.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 42.Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381:697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- 43.Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol. 2000;84:301–310. doi: 10.1152/jn.2000.84.1.301. [DOI] [PubMed] [Google Scholar]
- 44.Theeuwes J, Kramer AF, Kingstone A. Attentional capture modulates perceptual sensitivity. Psychon Bull Rev. 2004;11:551–554. doi: 10.3758/bf03196609. [DOI] [PubMed] [Google Scholar]
- 45.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 46.Richmond BJ, Optican LM, Podell M, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J Neurophysiol. 1987;57:132–146. doi: 10.1152/jn.1987.57.1.132. [DOI] [PubMed] [Google Scholar]
- 47.Wilson DR, Martinez TR. Improved heterogenous distance functions. J Artif Intell Res. 1997;6:1–34. [Google Scholar]
- 48.Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- 49.Maunsell JH, Gibson JR. Visual response latencies in striate cortex of the macaque monkey. J Neurophysiol. 1992;68:1332–1344. doi: 10.1152/jn.1992.68.4.1332. [DOI] [PubMed] [Google Scholar]


