Abstract
Each time the eyes move, the visual system must adjust internal representations to account for the accompanying shift in the retinal image. In the lateral intraparietal cortex (LIP), neurons update the spatial representations of salient stimuli when the eyes move. In previous experiments, we found that split-brain monkeys were impaired on double-step saccade sequences that required updating across visual hemifields, as compared to within hemifield (Berman et al. 2005; Heiser et al. 2005). Here we describe a subsequent experiment to characterize the relationship between behavioral performance and neural activity in LIP in the split-brain monkey. We recorded from single LIP neurons while split-brain and intact monkeys performed two conditions of the double-step saccade task: one required across-hemifield updating and the other within-hemifield updating. We found that, despite extensive experience with the task, the split-brain monkeys were significantly more accurate for within-hemifield as compared to across-hemifield sequences. In parallel, we found that population activity in LIP of the split-brain monkeys was significantly stronger for within-hemifield as compared to across-hemifield conditions of the double-step task. In contrast, in the normal monkey, both the average behavioral performance and population activity showed no bias toward the within-hemifield condition. Finally, we found that the difference between within-hemifield and across-hemifield performance in the split-brain monkeys was reflected at the level of single neuron activity in LIP. These findings indicate that remapping activity in area LIP is present in the split-brain monkey for the double-step task and co-varies with spatial behavior on within-hemifield compared to across-hemifield sequences.
INTRODUCTION
Visual perception is based on both incoming sensory signals and information about ongoing actions. Evidence for this idea comes from physiological studies, which have demonstrated that motor signals can influence visual representations in parietal, frontal and extrastriate cortex, and in the superior colliculus (Duhamel et al. 1992a; Goldberg and Bruce 1990; Mays and Sparks 1980; Nakamura and Colby 2002; Walker et al. 1995; Umeno and Goldberg 1997, 2001). In each of these areas, visual representations are updated in conjunction with eye movements, as shown in the single-step saccade task. In this task, activity of a single neuron is monitored while the monkey makes a simple saccadic eye movement. This eye movement brings the neuron’s receptive field onto a location where a visual stimulus had previously appeared. The neuron fires, despite the fact that the stimulus is never physically in the receptive field. The firing represents a response to the memory trace of the stimulus, which has been updated to take the eye movement into account. Updating is presumed to involve a transfer of information from neurons that encode the stimulus location before the eye movement, to those that will encode its location after the eye movement. This updating activity provides a mechanism for creating a stable, eye-centered map of salient locations (Colby and Goldberg, 1999).
Our central hypothesis, investigated in a series of experiments, is that updating relies on the integrity of direct cortico-cortical links. We have tested this hypothesis by examining a case in which these cortical connections are accessible to experimental manipulation. We compared updating for stimulus traces that remain within a single hemifield to updating for stimulus traces that must be transferred across hemifields (Fig. 1). In the across-hemifield case, a visual stimulus is presented briefly in one hemifield. Following a saccadic eye movement, the trace of that stimulus is brought into the opposite hemifield. Consequently, visual representations that originate in one cortical hemisphere must be transmitted to the opposite hemisphere. The forebrain commissures – the corpus callosum and the anterior commissure – comprise the only route for direct communication between the cortical hemispheres. In two previous reports, we described separate behavioral and physiological experiments that test whether the forebrain commissures are required for interhemispheric updating (Berman et al. 2005; Heiser et al. 2005). We found that direct cortical links are an important substrate for spatial updating but are not strictly necessary. While split-brain animals showed an initial behavioral impairment on a task that requires across-hemifield updating, performance improved substantially with experience. At the single neuron level, neurons in the lateral intraparietal area (LIP) were active for both across- and within-hemifield updating, with reduced activity in the across-hemifield case.
Fig. 1.
Assessment of behavior and neural activity during within- and across-hemifield remapping in the split-brain monkey. The geometry of the double-step sequence is determined by the location of the neuron’s receptive field. The hypothetical neuron under study is located in the left hemisphere (grey asterisk), with a receptive field (circle) in the upper right visual field. In the across-hemifield condition (A), the second target (T2) is located in the left visual field when the eyes are at central fixation (FP); its location is represented by neurons in the right hemisphere (black asterisk). When the eyes reach the first target (T1), however, the location where T2 appeared is now in the right visual field; its stimulus trace is thus represented by neurons in the left hemisphere, including the neuron under study (gray asterisk). Updating in this condition involves a transfer of visual information between neurons in opposite cortical hemispheres. In the within-hemifield condition (B) T2 appears in the right visual field when the eyes are at FP, and so is represented by neurons in the left hemisphere (black asterisk). After the saccade to T1, the stimulus trace of T2 is still represented by neurons in the left hemisphere (gray asterisk). Updating in this condition therefore involves the transfer of visual signals within the same hemisphere.
The differences that we observed between within-hemifield and across-hemifield remapping in the split-brain monkey present an unusual opportunity to examine the relationship between updating activity and spatial behavior. In the current study, we investigated this relationship using the double-step saccade task, which allowed us to measure simultaneously the neural activity and behavior associated with remapping. The double-step task gives insight into the ability to monitor and adjust for intervening gaze shifts (Baker et al. 2003; Baizer and Bender 1989; Duhamel et al. 1992b; Goldberg and Bruce 1990; Hallett and Lightstone 1976; Mays and Sparks 1980; Mazzoni et al. 1996; Murthy et al. 2007; Ray et al. 2004; Sommer and Wurtz 2002; Vliegen et al. 2004). In this task, subjects make successive eye movements from a central fixation point (FP) to two targets, T1 and T2. The second target, T2, disappears before the eyes begin to move. This creates a mismatch between the initial retinal representation of T2 and the ultimate motor vector needed to acquire T2. For the subject to perform the sequence accurately, the representation of T2 must be updated to take the first saccade into account. Updating activity could be the mechanism for solving the spatial integration problem posed by the double-step task. Neurons in area LIP become active when the saccade to T1 brings the receptive field onto the location where T2 had appeared (Goldberg et al. 1990). This activity reflects a representation of the second target that takes the first saccade into account: T2 is encoded in coordinates that specify the vector needed to acquire T2 from the eyes’ new position at T1.
In order to test the relationship between spatial updating activity and behavior, we recorded from single neurons in area LIP of split-brain and intact monkeys while they performed two conditions of the double-step task. In the across-hemifield condition, the representation of the T2 must be updated from one visual hemifield to the other, requiring interhemispheric communication of visual information (Fig. 1A). In the within-hemifield condition, the representation of T2 must be updated from one location to another, within the same hemifield (Fig. 1B).
This study had three objectives. The first was to determine whether split-brain monkeys exhibit a selective impairment in the performance of across-hemifield sequences during physiological recording sessions, even after extensive previous training and testing on the double-step task. The second objective was to determine whether activity in area LIP of the split-brain monkey is altered for double-step sequences that require across-hemifield as compared to within-hemifield updating. Our third objective was to investigate the correspondence between physiological and behavioral measures of spatial updating in the split-brain monkey.
METHODS
General procedures
Subjects were three rhesus macaques. The forebrain commissures were intact in monkey FF; in monkeys EM and CH, the forebrain commissures were surgically transected at the outset of the experiments (Berman et al. 2005; Heiser et al. 2005). The commissurotomy is described in detail elsewhere (Vogels et al. 1994). Briefly, the monkeys were prepared for this surgery with dexamethasone, and anesthesia was induced with ketamine and maintained with isoflurane. Mannitol was administered throughout the surgery to minimize tissue swelling. The corpus callosum was transected along its full length using a small glass pipette with suction; the anterior commissure was fully transected. In the two weeks following the surgery, analgesics and antibiotics were administered daily.
Following completion of behavioral training and testing, monkeys were prepared for chronic physiological recording. The placement of the recording chamber (1.8 cm diameter) was determined using anatomical information from structural magnetic resonance images in conjunction with the standard stereotaxic locations for area LIP (5mm posterior and 12mm lateral in Horsley Clarke coordinates). We used MRI to verify that the chambers were located over the intraparietal sulcus. Recording began 14–21 months after the commissurotomy, and 7–11 months after the start of behavioral testing described in Berman et al. 2005. Double-step physiology data for the split-brain monkeys were collected in parallel with the single-step physiology described in Heiser et al. 2005. Animals were cared for and handled in accordance with NIH guidelines, and all experimental protocols were approved by the University of Pittsburgh Institutional Animal Care Use and Committee.
During recording sessions, the monkey sat in a darkened room with its head fixed in a primate chair, facing a tangent screen. Visual stimuli were back-projected on the tangent screen using a LCD projector. Stimulus presentation was under the control of two computers running a C-based program, CORTEX, made available by Dr. Robert Desimone. Eye position was monitored using scleral search coils (Judge et al. 1980), with a sampling rate of 250 Hz.
Physiological Methods
Neural activity was recorded using tungsten microelectrodes (Frederick Haer, Bowdoinham, ME) introduced into the cortex through stainless steel guide tubes placed flush with the dura. The guide tubes were stabilized by a nylon grid (Crist Instruments) held rigidly in the recording chamber. The grid system permitted parallel penetrations along the bank of the intraparietal sulcus (IPS) with a resolution of 1 mm. Action potentials were amplified and filtered with a band-pass of 500 Hz to 5 kHz, and digitally sampled using template matching at 20 kHz. Individual neurons were isolated by means of an on-line spike-sorting system using a template-matching algorithm (Signal Processing Systems, Prospect, Australia).
Behavioral Paradigms
Memory guided saccade task
We used the memory guided saccade task to search for neurons and assess their visual, memory and saccade-related response properties. In this task, the monkey began by fixating on a central fixation point. After an initial delay of 300–500 ms, a stimulus appeared in the receptive field for 50 ms. After a second delay of 400–800 ms, the fixation point was extinguished, which cued the monkey to make a saccade to the location of the flashed stimulus. After the saccade, the stimulus reappeared and the monkey maintained fixation on it for an additional 300–500 ms. We used standard mapping procedures to determine the location of a neuron’s receptive field (Barash et al. 1991).
Double-step task
This task provides a measure of both the neural activity and behavior associated with spatial updating. At the start of each trial, the monkey maintained fixation on a central fixation point (FP) for 300–500ms. The first saccade target (T1) then appeared and remained illuminated. The second target (T2) appeared 100 ms later, and was extinguished after 50 ms. The fixation point was extinguished simultaneously with the disappearance of T2, cueing the monkey to initiate the double-step sequence. The monkey made a visually-guided saccade to T1 (T1 was extinguished upon completion of the first saccade), followed by a memory-guided saccade to T2. The second target then re-appeared, and the monkey fixated this target location for an additional 300–500 ms before receiving juice reward.
Single-step task
Stimuli in the single-step task have the same configuration as the double-step task. The important difference is that in the single-step task, the stimulus to be updated is not the target of an eye movement, and is irrelevant to the monkey’s behavior. The monkey attained central fixation and maintained gaze there for 300–500 ms. Two events then occurred simultaneously: a peripheral stimulus appeared outside of the neuron’s receptive field, and a new fixation point (T1) was illuminated. The peripheral stimulus was extinguished 50 ms later, simultaneously with the disappearance of FP. This was the monkey’s cue to make a visually guided saccade to T1. The saccade to T1 moved the neuron’s receptive field onto the location of the now-extinguished stimulus. The monkey maintained gaze on T1 for an additional 500–700 ms to receive juice reward.
Saccade control task
This visually-guided saccade task was used to determine whether activity in the single-step task could be attributed to the generation of the saccade alone. Task events and timing were identical to the single-step task, except that no peripheral stimulus was presented.
Stimulus control task
The stimulus control task was used to ensure that the stimulus location used in the single-step task was outside of the receptive field and did not drive the neuron. In this task, the monkey maintained central fixation for 300–500 ms. The peripheral stimulus was flashed for 50 ms, and the monkey was required to maintain fixation for an additional 1200–1500 ms.
Experimental design
We initially assessed neurons in the MGS task, and then began the experimental protocol. This protocol consisted of eight types of trials for each neuron: four tasks (stimulus control, saccade control, single-step and double-step) x two conditions (within-hemifield, across-hemifield). We collected 12–20 trials for each trial type. The tasks were run in separate blocks, always in the same order: stimulus control, saccade control single-step, double-step. We collected data in this order because previous experiments have demonstrated that long-term inter-trial memory responses can persist after experience with updating tasks (single-step or double-step) and can lead to activity in subsequent saccade control trials (Umeno and Goldberg 2001). In each block, the within and across conditions were randomly interleaved. This interleaving was critical to the design, as each neuron served as its own control.
The exact geometry of the within-hemifield and across-hemifield conditions was tailored for each neuron, based on the location of the receptive field (Fig. 1). By definition, different spatial configurations are required for remapping stimulus traces within and across hemifields. We held saccade amplitude constant and varied only the direction of the first saccade of the within and across conditions. The second saccade always had the same direction and amplitude for the two conditions, as it was described by the vector between central fixation and the neuron’s receptive field. We used two standard configurations for most neurons. In the within-hemifield condition, a vertical saccade kept the representation of the second target within the same hemifield both before and after the first saccade. In the across-hemifield condition, a horizontal ipsiversive saccade moved the representation of T2 from one hemifield to the other. For the remaining neurons, we used diagonal saccades for one or both conditions. For the first saccade, average amplitude was 21.3° (± 3.4° s.d.). For the second saccade, average amplitude was 15.1° (± 5.1° s.d.).
Analysis
Analysis of double-step saccade data
We quantified the accuracy of the second saccade by calculating distance error, which describes the absolute difference between the saccade endpoint and the target. Latency of the first saccade was defined as the time between the disappearance of the central fixation point and the initiation of the first saccade (velocity above 50°/s). Latency of the second saccade was defined as the time between the end of the first saccade (velocity below 20°/s) and the initiation of the second saccade.
Selection of double-step saccade data
We screened the behavioral data at two stages of analysis in order to ensure that the neural and behavioral findings were representative and uncontaminated by inattentive performance of the task. First, for an individual trial to be considered valid, the latency of the first saccade had to be between 50 and 350 ms, and this saccade had to reach the first target accurately. We assessed accuracy using a measure of saccade gain, where gain had a maximum value of one, and was equal to the absolute difference between target amplitude and distance error, divided by target amplitude. We required that first-saccade gain be at least 0.75 for each individual trial (upper limit of the gain measure was, by definition, 1) . Second, the average saccade data associated with a given neuron (a “session”) were included only if there were a minimum of 10 valid trials for each condition (of the 12–20 total trials collected), with an average first-saccade gain of at least 0.85 for each condition. In the final datasets for split-brain and intact animals, the accuracy of the first saccade was not significantly different for the within-hemifield and across-hemifield conditions (p > .05, Wilcoxon signed rank). Therefore, conditional differences in the accuracy of the second saccade could not be attributed to differences in the first saccade. The landing point of the second saccade is the critical measure, as correct performance requires spatial updating; the first saccade was visually guided and could be completed using simple retinal information. We tested for significant differences between within- and across-hemifield behavior using the nonparametric Wilcoxon signed rank test.
Selection of neurons
Our initial database (n = 277) included all single neurons recorded in the lateral intraparietal sulcus that exhibited a significant visual response in the memory-guided saccade task (t-test comparing a visual epoch, 100ms following the onset of the stimulus response, and a baseline epoch, 200–300ms after attainment of fixation). Neurons were then excluded on the basis of insufficient behavioral data (see above). Our remaining physiological criteria focused on ensuring a clear interpretation of activity in the single-step and double-step tasks. In these tasks, updating activity occurs when the monkey makes a saccade to the first target (T1), which brings the memory trace of the stimulus into the receptive field. Updating activity is not attributable either to the stimulus alone or to the saccade alone. It was therefore critical that we take into account any activity due simply to the stimulus or saccade alone. We adjusted for saccade-alone activity using a subtraction method, described below. For stimulus-alone activity, we analyzed the activity in the stimulus control task to be certain that the response in the single-step task could not be attributed to the presence of the stimulus alone. We compared firing rate in a visual epoch of the stimulus control task (50–250 ms after stimulus onset) to that in the baseline epoch (200–300 ms after fix attain). We excluded neurons if they had a significant visual response in either stimulus control condition (t-test, p < 0.05). Approximately one quarter of all LIP neurons recorded had such a response – in other words, the receptive fields were large enough that the to-be-remapped stimulus evoked a response even when the monkey was fixating centrally. These cells were not analyzed further. Our final database was comprised of 181 cells with sufficient behavioral data and no significant activity in the stimulus-alone control.
Assessment of updating activity
We use the term ‘updating activity’ to refer to the neuron’s response to a stimulus trace that has been updated in conjunction with an eye movement. We measured updating activity in the epoch beginning at the initiation of the first saccade, and ending at the initiation of the second saccade. This epoch was computed individually for each trial of the double-step task. On average, the duration of this epoch was 174ms (s.d. = 34ms). We chose this epoch so that remapping was measured during a time-window that was identical from trial to trial with respect to the eye movements. If, for example, we had measured remapping solely in relation to the second saccade, the remapping epoch would include variable amounts of time before or after the first saccade. We therefore measured remapping in relation to both S1 and S2 to minimize the effects of variability in first saccade latencies. It is important to note that, although the epoch is variable in its duration, the measure of firing rate (spikes per second) is inherently independent of epoch duration.
It is essential that our measure of updating activity does not reflect firing related simply to the presence of the stimulus alone or to the saccade alone. As described above, we excluded at the outset any neurons that had a significant response to the stimulus alone. A number of the included neurons exhibited some response in the saccade control task. This activity typically occurred for spatial configurations in which the saccade alone (saccade to T1) brought the neuron’s receptive field onto the location where the central fixation point had appeared. Given this and earlier observations in our lab, the most parsimonious explanation for this activity in the saccade-alone task is that it represents remapping of the fixation point (Heiser and Colby, 2005). We adjusted for this saccade-alone activity by computing the average firing rate in the saccade-alone task, in an epoch that was identical to the average double-step remapping epoch for the individual neuron. We did this for all neurons, for both the within and across conditions, regardless of whether there was significant activity in the saccade-alone task. The average double-step remapping epoch was computed separately for within and across conditions, because each condition required a different first saccade. This computation ensured that the saccade-alone epoch for each condition corresponded to the same time window as used for the double-step task. For example, if a neuron had an average across-hemifield remapping epoch of 190ms (beginning at the start of the first saccade), then the across-hemifield saccade-alone epoch was also 190ms, beginning at the start of the first saccade. We report updating activity as the average firing rate in the double-step remapping epoch minus the average firing rate in the corresponding epoch of the saccade control task. Throughout the paper, the phrase “updating activity” refers to this adjusted firing rate. If activity in the saccade control exceeded activity in the double-step task, updating activity takes on a negative value. Updating activity in the double-step task was deemed significant when the firing rate in the remapping epoch exceeded that in the corresponding saccade control epoch at a significance level of p < .05 (t-test).
We computed a neural Within:Across (WA) index to quantify the strength of the neuron’s preference for either the within-hemifield or across-hemifield condition. As described previously (Heiser et al. 2005), this index normalizes the updating activity observed in the double-step task to the total activity observed in the double-step and saccade control tasks, using the following formula: WA Index = (DSw−SACw)−(DSa−SACa)/(DSw+SACw)+(DSa+SACa). In this formula, DSw and DSa represent the firing rates measured in the within and across conditions of the double-step task, and SACw and SACa represent the firing rates measured in the corresponding saccade control conditions. The denominator of this formula accounts for the fact that the saccade-alone activity exceeded double-step activity for at least one condition in some neurons. The formula ensures that the index has a range of −1 to +1. Positive values indicate that activity was stronger for within-hemifield updating, whereas negative values indicate that activity was stronger for across-hemifield updating.
We used two analyses to assess the effect of task (double-step versus single-step) on the strength of LIP activity. In the first, we compared average firing rates directly. Activity in the single-step task was measured in a remapping epoch, aligned on the start of the first saccade. The duration of this epoch was identical to the average epoch used for the double-step task for each condition (within and across). We directly compared the double-step and single-step firing rates, without subtracting any saccade control activity, as the contribution of saccade-alone activity would be equivalent for the two tasks. The same logic applied for our second analysis, where we also used average firing rates without subtracting saccade control activity. In this second analysis, we measured the average difference between within-hemifield and across-hemifield activity for each of the tasks (single-step and double-step) and then compared the within:across differences for the two tasks.
Measuring neural latency
We measured the latency of the remapped response relative to the beginning of the first saccade. Neural latency can only be reliably defined with the method described below if all of the activity present in the double-step task is attributable to remapping the stimulus, rather than simply to the generation of the saccade. In contrast to the analysis of the strength of the remapped responses described above, there was no method to account for saccade control activity in the analysis of neural latency. Therefore, if there was any significant activity in the saccade control associated with a particular double-step condition, we excluded it from latency analyses.
Previous studies have shown that remapping can occur over a broad range of latencies (Duhamel et al. 1992a; Umeno and Goldberg 1997). We used the following method to measure neural latency in individual neurons (Nakamura and Colby 2000; Heiser et al. 2005). We searched for the onset of the neural response in the time window from 100 ms before saccade onset to 300 ms after saccade onset. We used a sliding window to find the time when the firing rate first began to differ significantly from activity during the baseline epoch (200–300 ms after attainment of fixation). Specifically, we measured activity in a 20 ms response window beginning 100 ms before saccade onset. We used a t-test (p < 0.05) to assess whether activity in the response window differed significantly from baseline activity. If there was no significant difference, the window was shifted forward by 2 ms, and the procedure was repeated until the activity in the response window was significantly greater than baseline activity. In order to avoid spurious results, we defined latency based on the occurrence of two consecutive bins that achieved significance. The midpoint of the first bin was considered the onset of the neural response. If this criterion was not met within 300 ms after saccade onset, we concluded that there was no response associated with remapping the stimulus trace. We used an analogous method to determine the visual response latency in the memory guided response task. The calculated latency was verified by inspection. For all matched comparisons of neural activity (within-hemifield versus across-hemifield, single-step versus double-step) or neural latency, we used the Wilcoxon signed rank test.
Trial-by-trial analysis in single neurons
Our assessment of the relationship between neurons and behavior included an analysis of the trial-by-trial correlation between updating activity in single neurons and double-step saccade performance. As stated above, one of the challenges in measuring updating activity is that we must remove the contributions of saccade-alone activity. When we consider individual trials, there is no principled way to match individual saccade-alone trials to individual double-step trials for this subtraction. We used the following method to remove the contributions of saccade-alone firing from double-step activity on single trials. For a given neuron, we computed the double-step firing rate for each trial as described above, measuring the spikes per second in the epoch from the beginning of the first saccade to the beginning of the second saccade. From each individual-trial double-step firing rate, we then subtracted the average saccade-alone firing rate for that condition (within or across). The average saccade-alone firing rate was computed using the epoch corresponding to the average double-step epoch for that neuron and condition. This method allowed us to remove the contributions of saccade-alone activity while maintaining information about updating activity on individual trials. We assessed the relationship between updating activity and behavior (accuracy or latency) by performing a Pearson’s correlation analysis.
RESULTS
The goal of these experiments was to characterize the behavioral and physiological correlates of spatial updating in split-brain monkeys. We recorded from a total of 277 single neurons in the lateral intraparietal cortex of two split-brain monkeys (n=216) and an intact monkey (n=61) during performance of this task. Of these, we describe the neural activity and associated saccadic data from recording sessions that met a series of physiological and behavioral criteria (see Methods). The results focus on a final dataset of 139 neurons from the split-brain animals, and 42 neurons from the intact animal. We monitored each neuron’s activity while the monkeys performed two conditions of the double-step saccade task, which required spatial representations to be updated either across or within visual hemifields (Fig. 1). The results are presented in three sections. In the first section, we characterize the behavioral performance of the split-brain and intact monkeys during these physiological recording sessions. In the second section, we describe the accompanying neural activity in area LIP. This section focuses on the comparison between within-hemifield and across-hemifield remapping, and subsequently addresses the comparison of the single-step and double-step tasks. In the third section, we investigate the relationship between behavior and neural activity.
I. PERFORMANCE ON THE DOUBLE-STEP TASK
In behavioral studies described previously, we discovered that split-brain monkeys were initially impaired in performance on a set of standard across-hemifield double-step sequences, but were ultimately able to perform these sequences well (Berman et al. 2005). Nonetheless, the monkeys’ performance of across-hemifield sequences remained less accurate than that of within-hemifield sequences. This inaccuracy was most evident when we introduced a novel spatial configuration. In the recording sessions described here, the spatial configuration of the task was necessarily determined by the location of the response field of each neuron. As a result, the monkeys were commonly presented with new spatial configurations of the double-step task. We therefore predicted that, during physiological recording, the split-brain monkeys would continue to demonstrate a behavioral impairment for the across-hemifield sequences as compared to the within-hemifield sequences.
Performance of across-hemifield sequences is moderately compromised in the split-brain monkey
We found that the split-brain monkeys were selectively impaired in the across-hemifield condition of the double-step saccade task during physiological recording sessions. An example of double-step performance during recording from a single neuron is shown in Fig. 2. The split-brain monkey was very accurate on the within sequence (A) but less accurate on the across sequence (B). For this and every recording session, we quantified the accuracy of the monkeys’ double-step performance by computing the distance error of the second saccade, using all trials in which the monkey accurately reached the first saccade target (T1).
Fig. 2.

Activity in a single neuron that remaps both within and across hemifields in the double-step task. The monkey makes sequential saccades from fixation (crosshair) to the first target (dot) and second target (asterisk). The first saccade brings the neuron’s receptive field onto the location where the second target had appeared. Eye position traces from the first ten trials are shown for the within (A) and across (B) conditions. Recording during the double-step task shows neural activity during the within (C) and across (D) conditions. Recording during the single-step task likewise shows that activity in both the within (E) and across (F) conditions is greater than in the control tasks (G–J).
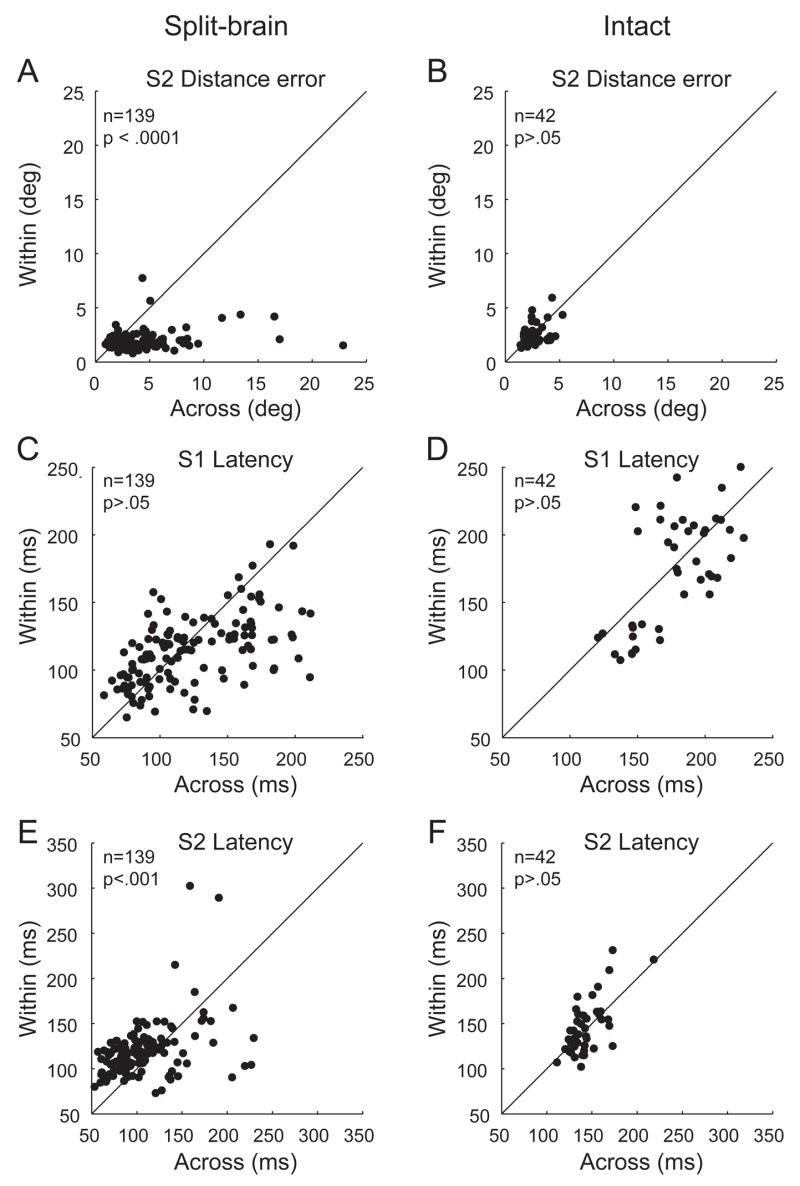
The behavioral results from all recording sessions were consistent with the pattern of double-step performance seen in Fig. 2. On average, error on the second saccade was significantly greater for the across as compared to the within condition (Fig. 3A: across error = 3.90 ± 0.26° (SE); within error = 1.92 ± 0.077°, p < .0001, Wilcoxon signed rank test). We observed no significant difference between within- and across-hemifield accuracy in the intact monkey (Fig. 3B: across error = 2.67 ± 0.14; within error = 2.54 ± 0.15°, p > .05, Wilcoxon signed rank).
Fig. 3.
Accuracy and latency of double-step performance. Panels in the left column represent data from the two split-brain monkeys; those in the right column are from a monkey with commissures intact. Each point represents average double-step behavior during the recording of a single neuron, for the within-hemifield (y axis) and across-hemifield condition (x axis). Distance error of the second saccade (S2) was significantly greater for across-hemifield updating in the split-brain (A) but not the intact animal (B). For latency (C–F), both the first and second saccades were faster overall for the split-brain monkeys, who were highly experienced on the task as compared to the intact monkey. For the first saccade (S1), within-hemifield and across-hemifield latencies were not significantly different for either split-brain or intact (C,D). For the second saccade, overall latencies were significantly faster for the across-hemifield condition in the split-brain (E) but did not differ in the intact monkey (F).
We next asked whether impairment of the across-hemifield sequences would also produce prolonged saccadic latencies. In our initial behavioral testing on a set of standard sequences, the split-brain monkeys exhibited moderate increases in latency on the first and second saccades of the across-hemifield sequences. By the end of many months of behavioral testing, however, the monkeys were no longer delayed in saccade initiation in the standard across-hemifield sequences (Berman et al. 2005). Further, across-hemifield latencies were not consistently prolonged when we introduced a new spatial arrangement of the double-step targets, even though accuracy could again be impaired. We therefore predicted that across-hemifield latencies would not be slowed during the physiological recording sessions.
In the present study, we indeed found that saccadic latencies were not uniformly prolonged for the across-hemifield as compared to the within-hemifield condition, in either the split-brain or intact animal (Fig. 3C–F). In the split-brain monkeys, average S1 latencies were slightly prolonged for the across-hemifield condition (Fig. 3C, 123.8 ± 3.3 ms (SE), compared to 115.9 ± 2.1 ms for within-hemifield). This difference approached but did not reach significance (p = 0.06, Wilcoxon signed rank). By contrast, average S2 latencies were significantly faster for the across-hemifield condition (Fig. 3E, 108.4 ± 3.1ms, compared to 120.3 ± 2.8ms for within-hemifield; p < .001, Wilcoxon signed rank). This observation is in keeping with our findings at the end of the initial behavioral testing: S2 latencies for the across-hemifield condition were no longer delayed, and were actually faster than those for the within-hemifield condition (Berman et al. 2005). In the intact monkey, we found no significant differences between within- and across-hemifield saccade latencies for either saccade (Fig. 3D,F: S1 across: 177.5 ± 4.5 ms, S1 within: 174.1 ± 6.3 ms, p = .29; S2 across: 143.6 ± 2.9 ms, S2 within: 145.8 ± 4.5 ms; p= .70, Wilcoxon signed rank). Overall, the split-brain monkeys had faster average saccade latencies than the intact monkey. This may reflect the very extensive experience of the split-brain monkeys on the double-step task. Each of the split-brain monkeys had more than 1.5 years of experience with the task before recording began. In contrast, the intact monkey had less than six months of experience.
These data show that S2 latencies in the split-brain monkeys were faster for across-hemifield as compared to within-hemifield sequences. We considered the possibility that this finding reflected a trade-off between speed and accuracy, in which faster saccade latencies were associated with greater error. Accordingly, we plotted average S2 accuracy and latency against each other for all recording sessions (Fig. 4). Correlation analyses showed that the faster latencies were slightly but significantly related to smaller errors, both in the split-brain monkeys (Fig. 4A, slope = .013, r = .18, p < .05) and in the intact monkey (Fig. 4B, slope = .010, r = .26, p < .05; Pearson’s correlation). This association between faster latencies and smaller errors remained significant when we focused solely on the across-hemifield condition for the split-brain monkeys (slope = .023, r = .27 p < .001). These findings indicate that the rapid latencies were not due to a speed-accuracy trade-off. In general, these very fast latencies likely reflect the fact that the split-brain monkeys were highly practiced as compared to the normal animal. The rapid across-hemifield latencies in the split-brain monkeys further suggest that they may have adopted a more automatic strategy for performing these sequences as they gained experience. In summary, the present findings demonstrate that the split-brain monkeys were only moderately impaired on performance of the across-hemifield double-step sequences. The monkeys exhibited greater errors for new across-hemifield sequences, though these errors were smaller than those observed in initial behavioral testing on the standard sequences (Berman et al. 2005). Latencies were not systematically prolonged for new across-hemifield sequences and were in fact faster in the case of the second saccade. These behavioral data reinforce the conclusion that spatial representations can be updated effectively across hemifields in the absence of the forebrain commissures.
Fig. 4.

Relationship between speed of saccade initiation and accuracy for the second saccade of the double-step task. Each point represents the spatial error (y axis) and average saccade latency (x axis) from a given neural recording session. Each session contributes two datapoints, one from within-hemifield trials and one from across-hemifield trials. The regression line is indicated by the thin black line. For both the split-brain (A) and intact (B) monkey, the overall slope of the relationship between speed and accuracy is positive: as latency increased, so did spatial error.
II. NEURAL ACTIVITY DURING PERFORMANCE THE DOUBLE-STEP TASK
Within-hemifield and across-hemifield updating
LIP neurons in the split-brain monkey remap stimulus traces across hemifields in the double-step task
Our second objective was to determine whether neurons in area LIP can remap spatial representations across visual hemifields in the double-step task in the absence of direct links between the cortical hemispheres. At the outset of our experiments in the split-brain monkey, our expectation was that LIP neurons would not exhibit remapping when the stimulus trace needed to be updated from one visual field to another. Results from recording during the single-step task, however, revealed that updating signals were present in area LIP of the split-brain monkey, even for the across-hemifield case (Heiser et al. 2005). We therefore expected that across-hemifield remapping would likewise be present in the double-step task.
Our central observation here is that neurons in area LIP of the split-brain monkey can update stimulus representations both within and across visual hemifields in the double-step saccade task. An example of this activity is shown for a single neuron in Figure 2. In the double-step task, the monkey made sequential saccades to two targets, T1 and T2 (Fig. 2A,B). The neuron responded vigorously for both the within-hemifield and across-hemifield conditions of this task (Fig. 2C,D). The neuron’s response was negligible in the corresponding stimulus-alone (Fig. 2G–H) and saccade-alone control tasks (Fig. 2I–J). The minimal activity in these control tasks demonstrates that activity in the double-step task is not attributable to the generation of the saccade alone, or to the presentation of the T2 stimulus alone. Rather, the activity represents the cell’s response to a stimulus trace of T2, which has been remapped in conjunction with the saccade to T1. This single neuron responded not only when the stimulus trace was updated within the same hemifield, but also when it was updated across hemifields. We asked whether this observation was evident in the population of LIP neurons, focusing on the prevalence, magnitude, and latency of updating activity.
Most lip neurons exhibit both within-hemifield and across-hemifield remapping
We first assessed the likelihood of observing significant updating activity in the across-hemifield condition of the double-step task (Fig. 5). We expected that across-hemifield remapping might be less prevalent than within-hemifield remapping in the absence of the forebrain commissures. In the split-brain monkeys, we found that 85% of neurons (119/139) exhibited significant remapping in at least one condition. Of these neurons, the vast majority had significant updating activity in both the across-hemifield and within conditions (70%, n=83, Fig. 5A). Some neurons had a significant response only for the within-hemifield condition (24%, n=29), and a few were significant for the across-hemifield condition alone (6%, n=7). In the intact monkey, we found that the majority of neurons exhibited significant remapping in at least one condition (64%, 27/42, Fig. 5B). Of these, the majority had significant updating activity in both conditions (56%, n=15). The remaining neurons were more likely to show significant remapping for the across-hemifield condition only (37%, n=10) than for the within-hemifield condition only (7%, n=2). The salient observation here is that, among neurons with significant remapping, a substantial majority of neurons had significant across-hemifield remapping in both split-brain and intact animals (76% and 92%, respectively). We conclude that even in the absence of the forebrain commissures, the majority of LIP neurons can respond to the updated representations of T2 that originate in the opposite hemisphere.
Fig. 5.

In the split-brain monkey, updating activity in LIP is stronger for the within-hemifield than the across-hemifield condition. This difference was not observed in the intact monkey. Bars represent the percentage of neurons with significant remapping for Within only, Across only, or both conditions in the split-brain (A) and intact monkey (B); neurons with no significant remapping are excluded. For all single neurons in the split-brain (C) and intact monkey (D), average updating activity in the within-hemifield condition (y axis) is plotted against that in the across-hemifield condition (x axis). Updating activity is adjusted to take saccade-alone activity into account (see Methods); negative updating activity (below or to the left of the dotted lines in C,D) indicates that saccade-alone activity exceeded activity in the double-step task.
Within-hemifield remapping is stronger than across-hemifield remapping in the split-brain monkey
We next asked whether the magnitude of remapping activity in the double-step task was similar for across-hemifield and within-hemifield conditions. The example neuron from the split-brain monkey (Figure 2) fired strongly in both conditions of the double-step task, though less for the across-hemifield condition. Our data from single-step experiments in these split-brain monkeys demonstrated that LIP activity was stronger for within-hemifield updating (Heiser et al. 2005). In the present study, we observed the same pattern for updating activity in the double-step task. On average, neurons in the split-brain monkey exhibited significantly stronger updating activity for the within-hemifield condition as compared to the across-hemifield condition (Fig. 5C; within-hemifield: 18.2 ± 1.5 sp/s (SE); across-hemifield:, 13.12 ± 1.3 sp/s; p < .0001, Wilcoxon signed rank). No difference was observed in the intact monkey (Fig. 5D; within-hemifield: 4.57 ± 0.95 sp/s; across-hemifield:, 5.98 ± 1.2 sp/s, p = .14, Wilcoxon signed rank). These data indicate that across-hemifield remapping, while clearly present in the split-brain monkey, is less robust than within-hemifield remapping.
We considered whether the difference between across-hemifield and within-hemifield activity in the split-brain monkey might reflect a difference in anticipated reward. Activity in area LIP may be modulated by reward or motivational signals (Dorris and Glimcher 2004; Platt and Glimcher 1999; Sugrue et al. 2004). In the present experiment, the split-brain monkeys performed the across-hemifield double-step sequences less accurately, and thus were rewarded less frequently on these trials. We reasoned that if reward modulation were responsible for the difference between within-and across-hemifield updating in the double-step task, then the difference would disappear if reward amounts did not differ. We analyzed a subset of neurons recorded in sessions where the split-brain monkeys received reward on at least 90% of trials, for both the within- and across-hemifield sequences. For this subset (n=22), there was no significant difference in the amount of reward received for the two conditions (p = .29, Wilcoxon signed rank test). There was, however, a significant difference in updating activity between conditions (within-hemifield: 26.9 sp/s; across-hemifield, 21.4 sp/s, p < .05, Wilcoxon signed rank). We conclude that stronger within-hemifield updating in the split-brain monkeys is not attributable simply to differential reward.
Latency of remapping in the within- and across-hemifield conditions
In our earlier study of the split-brain monkey, we found that across-hemifield updating activity began later than within-hemifield updating in the single-step task (Heiser et al. 2005). Might the same be true in the double-step task? We investigated this question in a subset of single neurons that had detectable neural latencies in both the within and the across condition. In order to detect the latency of updating accurately, it was necessary that the neurons have no significant saccade-alone activity for either condition, as this would interfere with the measure of the onset of updating activity. In the subset of neurons that met these criteria for both conditions (split-brain, N=22; intact, N=14), we could directly compare the neural latencies for within-hemifield and across-hemifield updating in the double-step task.
We found that the onset of updating activity was significantly later for the across-hemifield condition as compared to within-hemifield in the split-brain monkey, but not in the intact monkey. For each neuron, we compared the latency of within-hemifield remapping to that of across-hemifield remapping (Fig. 6). Points that fall along the unity line indicate that remapping began at the same time for the two conditions. In the split-brain monkeys, most points fall below the line, indicating that across-hemifield remapping began later than within-hemifield remapping in single cells (Fig. 6A, p < .05, Wilcoxon signed rank). In the intact monkey, neural latencies did not differ significantly for within-hemifield and across-hemifield conditions (Fig. 6B, p = .54, Wilcoxon signed rank). It is worth noting that there are several neurons in both the split-brain and intact monkeys in which updating activity begins even before the start of the eye movement (unshaded areas in Fig. 6A,B). This presaccadic activity is an example of predictive remapping and provides an updated representation before the eye movement is initiated. In the split-brain monkeys, a small number of neurons exhibit presaccadic remapping even in the across-hemifield condition (dots to the left of the dashed vertical line), although they are less common than neurons that exhibit presaccadic within-hemifield remapping (dots below the dashed horizontal line). These neural latency data lend further support to the conclusions from our firing rate analyses: in the split-brain monkey, neural signals associated with across-hemifield updating are common but are modified relative to within-hemifield signals.
Fig. 6.

In the split-brain monkey (A), neural activity begins earlier for within-hemifield than across-hemifield updating. This difference was not observed in the intact monkey (B). Analysis was conducted on a subset of neurons with detectable neural latencies for both within and across conditions (see RESULTS). For each neuron, the neural latency in the within-hemifield condition (y axis) is plotted against that in the across-hemifield condition (x axis). Neural latency is determined relative to the beginning of the first saccade (S1). Grey shading represents the region in which both within-hemifield and across-hemifield neural latencies were after the start of S1; unshaded areas indicate neural activity that began even before the start of S1. This activity reflects predictive updating, which was more frequent in the split-brain monkey for the within than the across condition. This is consistent with delayed across-hemifield updating activity in the absence of the forebrain commissures.
Task-related differences in updating activity
Neural activity is stronger in the double-step task than in the single-step task
In the double-step task, LIP neurons update the representation of a stimulus that is highly relevant to the animals’ behavior – the stimulus is the target for the second saccade. Does this behavioral relevance influence neural activity? We addressed this question by comparing activity in the double-step and single-step tasks. In the single-step task, the monkey makes a single saccade from fixation to the first target (T1). This saccade brings the neuron’s receptive field onto the location where the stimulus had appeared. The stimulus, while salient due to its sudden onset, is not the target of the monkey’s second eye movement as it is in the double-step task.
We found that activity in LIP is influenced by behavioral demands: activity is clearly increased in the double-step task as compared to the single-step task. In Fig. 2, we show activity in the double-step (C,D) and single-step tasks (E,F) for the representative LIP neuron from a split-brain monkey. In both tasks, the neuron exhibited updating activity in the within-hemifield and across-hemifield conditions. What is striking is that the neuron’s activity increased sharply for the double-step task, irrespective of condition. We asked whether this task-related increase was also apparent in the population of LIP neurons. For each cell, we plotted average activity in the double-step task against activity in the single-step task for each of the conditions, for split-brain (Fig. 7A) and intact (7B) monkeys. The vast majority of points fall above the unity line, indicating stronger activity in the double-step task than in the single-step task. This increase was highly significant for the split-brain monkeys and for the intact monkey (p < .0001 for each group, Wilcoxon signed rank). These data indicate that activity is heightened when the stimulus to-be-updated is relevant for the monkey’s behavior.
Fig. 7.

Comparison of neural activity in the single-step and double-step tasks. For the split-brain (A) and intact animal (B), average firing rate is greater in the double-step as compared to the single-step task. Each dot represents the average firing rate for a single neuron for the double-step task (y axis) plotted against that for the single-step task (x axis). Each neuron contributes two data points, one for within and one for across. For the split-brain (C) and intact monkey (D), increased activity in the double-step task is present for both within-hemifield and across-hemifield updating. Each point represents the differential activity for the two tasks (Double-step minus Single-step, in sp/s). The average differential activity for each neuron is shown for the within condition (y axis) and across condition (x axis). The shaded square shows the region in which double-step activity is greater than single-step activity for both within-and across- hemifield conditions.
Increased activity in the double-step task occurs for both updating conditions
We next asked whether this task-related increase in neural activity occurred differentially for the within-hemifield as compared to the across-hemifield condition. We reasoned that, in the split-brain monkeys, the behavioral relevance of the stimulus might be conveyed more strongly when updating took place within the same hemifield. If so, we would observe a larger task-related increase in activity for the within-hemifield condition. In order to test this possibility, we computed the difference between average double-step and single-step activity for the within-hemifield condition for each neuron, and compared this to the same difference computed for the across-hemifield condition.
We found that increased activity in the double-step versus single-step task did not occur preferentially for the within-hemifield condition. In Fig. 7C and D, we plotted the task-related difference in average firing rates from each cell, for the within condition (y axis) and across condition (x axis). If the task-related increase had been stronger for the within condition, more points would fall above the unity line. Instead, the points are centered on the unity line, and do not differ significantly by condition; this was true for the split-brain animals and for the intact animal (p= .34 and p = .27, respectively; Wilcoxon signed rank test). In other words, when the split-brain monkeys performed the double-step task, the activity of LIP neurons did not increase preferentially for the within-hemifield condition as compared to the across-hemifield condition. This finding indicates that the behavioral relevance of the stimulus can strengthen neural activity for both within-hemifield and across-hemifield updating in the split-brain monkey.
In summary, we made three key observations regarding neural activity in area LIP during the double-step task. First, the majority of LIP neurons in the split-brain monkey exhibit updating activity in the double-step task, even when the second target must be updated from one visual hemifield to the other. Second, across-hemifield remapping is less robust than within-hemifield remapping in the split-brain monkey, as evidenced in reduced magnitude and delayed onset of activity. Finally, in both the intact and split-brain monkeys, neural activity is increased for the double-step as compared to the single-step task, whether updating is within or across visual hemifields.
III. IS UPDATING ACTIVITY RELATED TO BEHAVIOR IN THE SPLIT-BRAIN MONKEY?
The goal of this final section is to determine whether there is a correspondence between updating activity and double-step performance. This possibility is of particular interest in the split-brain monkey, where we observed conditional differences in both neural activity and behavior. Our focus is the generation of the second saccade in the double-step task, which requires spatial updating. Specifically, we asked whether the accuracy or latency of this saccade is related to the updating activity that precedes it. We first investigate the relationship between neural activity and behavior at the level of the population, and then ask whether updating activity in single LIP neurons is related to spatial behavior.
Population activity in LIP corresponds to double-step performance
Our first analysis focused on the correspondence between the neural population and behavior. For this analysis, we characterized the relative bias toward within-hemifield or across-hemifield updating. We used indices that capture the differences in neural activity and behavior between within-hemifield and across-hemifield updating. The Within:Across neural index is computed for each cell, on the basis of updating activity in the remapping epoch (see Methods). The Within:Across behavioral indices are computed by taking the difference between measures of within-hemifield and across-hemifield performance (e.g., error across − error within) and dividing by the sum of the two. For all indices, values range from −1 to +1; this normalization allowed us to assess data from different sessions and monkeys on a comparable scale. Positive values always denote a bias for within-hemifield updating, manifest in stronger updating activity or greater accuracy or more rapid performance on the within-hemifield as compared to the across-hemifield condition. Using this approach, we asked whether the relative difference between within-hemifield and across-hemifield updating was similar for neural activity and behavior.
We observed key similarities between updating activity in the population of LIP neurons and overall behavior. In the split-brain monkey, the distributions for both the neural indices and the accuracy indices were skewed positively (p < .0001, sign test, Fig. 8A,C), indicating that the within-hemifield bias in LIP activity was aligned with the bias in spatial accuracy. The latency index, by contrast, was skewed negatively (p<.0001, Fig. 8E), representing the overall bias for faster saccade initiation in the across-hemifield as compared to the within-hemifield condition. In the intact monkey, the distributions of all three indices did not differ significantly from zero (Fig 8B,D,F; p>.05). When we compared the index distributions from the split-brain monkeys to those of the intact monkey, we found that the distributions differed significantly from each other on all three measures (neural indices, p<.0001, error indices, p<.00001; latency indices, p< .001; Wilcoxon rank sum test). These normalized indices confirm our earlier analyses and provide a concise synopsis of the similarities and differences between LIP population activity and average behavior.
Fig. 8.
Population measures of updating strength and behavioral accuracy and latency. Each panel shows the distribution of Within:Across (WA) index values from all neurons (single recording sessions). The index represents a bias for within-hemifield updating (positive values) or across-hemifield updating (negative values). The vertical line indicates no difference between within- and across-hemifield updating. In the split-brain monkey, the WA index is positively skewed for neural activity and distance error (A,C), but is negatively skewed for saccade latency (E). In the intact monkey, the WA index distribution does not differ from zero for neural activity (B), accuracy (D), or latency (F). Thus, at the population level, saccade accuracy parallels neural activity for both split-brain and intact monkeys. Saccade latency, by contrast, parallels neural activity in the intact monkey but not in the split-brain monkeys.
Trial-by-trial relationship between updating activity and behavior
Our second analysis emerges from the basic observation that the split-brain monkeys exhibited differences between within-hemifield and across-hemifield updating, in both activity and behavior. These differences in the split-brain monkey present an opportunity to ask whether the activity of single LIP neurons is related to performance of the double-step task. The objective of this second analysis is to determine whether remapping in single neurons bears any relationship to behavior on a trial-by-trial basis. For example, if a single neuron has stronger updating activity on certain trials than on others, is the monkey’s performance likely to be more accurate on those same trials? We examined this possibility by conducting a correlation analysis across trials. We asked whether the accuracy or latency of the second saccade was significantly related to updating activity on a trial-by-trial basis. For each neuron, we obtained the Pearson’s correlation coefficient, r, for the relationship between the saccade behavior (accuracy or latency) and updating activity (see Methods). We then assessed the distribution of r values from the population of LIP neurons. If remapping in single LIP neurons is systematically related to double-step performance on a trial-by-trial basis, then the distribution of r values will be significantly shifted away from zero.
We conducted this trial-by-trial analysis in two ways. In the first analysis, we analyzed the data from all trials, combining both within-hemifield and across-hemifield conditions. In the split-brain monkey we knew from preceding analyses (e.g., Figs 3 and 5) that there were systematic differences between the within and across conditions for updating activity and for performance. The first analysis allowed us to determine whether such differences in behavior and updating activity co-occur when measured at the level of single neurons. In the second analysis, we computed the trial-by-trial correlations separately for the within and across conditions. If we observed a relationship for a single condition, it would indicate that the trial-by-trial variability in updating activity was significantly linked to behavioral performance, irrespective of any overall differences between within- and across-hemifield updating.
When data from both within-hemifield and across-hemifield conditions were combined, we found that the strength of remapping in LIP neurons in the split-brain monkeys was significantly related to the accuracy of the second saccade. The distribution of r values for the population was skewed negatively and was significantly different from zero (Fig. 9A; mean r = −0.096, p<.01, sign test). When we considered individual neurons, we found that nearly 30% exhibited a significant trial-by-trial correlation between remapping strength and accuracy (39/139; shaded in black in Fig. 9A). Of these, the majority had a negative correlation (31/39). These findings from the split-brain monkey demonstrate that stronger updating activity was slightly but significantly associated with smaller errors in individual recording sessions. We expected, however, that this association emerged from the differences between the within and across conditions. Was a trial-by-trial relationship present, independent of the conditional differences?
Fig. 9.

Trial-by-trial analysis for single-neuron updating activity in LIP and the accuracy of double-step performance. Panels show the distribution of r values for the trial-by-trial correlation between updating activity and error of the second saccade. The left column shows data from the split-brain monkeys, right column from the intact monkey. Top row (A,B) shows data for all trials (within and across combined), middle row (C,D) shows data for within-hemifield trials only, and bottom row for across-hemifield trials only (E,F). Black shading indicates single neurons for which the trial-by-trial relationship was significant at p < .05. Vertical line indicates zero; x axis is identical for all panels. Negative r values indicate that greater updating activity was associated with smaller error. In the split-brain monkey, the population of r values had a significant negative skew only when all trials were combined (A), due to the differences between within and across-hemifield updating. The distributions were not significantly skewed for separate within (C) or across (E) trials in the split-brain monkey. In the intact monkey, there was no relationship between updating activity and second-saccade error on the double-step task whether trials were combined (B) or separate (D,F).
When we conducted the trial-by-trial analyses separately for the two conditions, we found no significant relationship between updating activity and second-saccade accuracy. Neither distribution of r values differed significantly from zero (within, Fig. 9C, mean r = −.007, p = .73; across, Fig. 9E mean r = −.009, p = .31, sign test). Further, only a small number of individual neurons exhibited a significant relationship (7/142 for within alone, 11/142 for across alone). These data indicate that, once conditional differences are removed, there is no systematic relationship between the trial-by-trial variability in updating activity in single LIP neurons and the monkeys’ accuracy on the double-step task.
In the intact monkey, we did not observe a significant trial-by-trial relationship between the strength of updating activity and the accuracy of performing the second saccade, whether the within and across data were combined or analyzed separately. When the two conditions were combined, the distribution of r values for trial-by-trial correlations did not differ significantly from zero (Fig. 9B, mean r = −.007, p= .64, sign test). A small proportion of individual neurons exhibited a significant correlation between remapping strength and accuracy (5/42, 12%), but these showed no trend toward a negative (n=3) or positive (n=2) correlation. The absence of a significant relationship for the combined data in the intact animal is not surprising. Saccade accuracy was considerably less variable in the intact animal than in the split-brain animals, where greater variability was introduced by the difference between within-hemifield and across-hemifield updating. As expected, when we analyzed data from the within and across conditions separately, the distributions of r values for the intact monkey were not significantly shifted from zero (within, Fig. 9D, mean r = −.036, p = .09; across, Fig. 9F mean r = .003, p = .88, sign test). These data constitute further evidence that trial-by-trial variability in updating activity is not significantly related to the accuracy of double-step performance.
We next examined the relationship between updating activity in single neurons and the latency of the second saccade in the double-step task. For the split-brain animals, the distribution of r values had a slight positive shift which approached significance when the within and across trials were combined (Fig. 10A; mean r = .062, p = .09, sign test). When we considered the strength of the correlation in individual neurons, we found that about a third of single neurons in the split-brain monkey showed a significant relationship between updating activity and S2 latency (41/139). Here, a majority had a positive correlation (30/41), indicating a link between stronger activity and longer latencies. This counterintuitive relationship likely reflects the general tendency for LIP neurons in the split-brain monkey to have weaker updating activity in the across-hemifield condition, while saccade latencies were often faster in this condition. Consistent with this interpretation, we found that there was no relationship between updating activity and latency once the conditional differences were removed. When we conducted the analyses separately, the distribution of r values did not differ significantly from zero for either condition (within, Fig. 10C, mean r = .013 p = .73; across, Fig. 10E, mean r = .057, p = .18, sign test). We conclude that there is no significant trial-by-trial relationship between updating activity and saccade latency in the split-brain monkey.
Fig. 10.

Trial-by-trial analysis for single-neuron updating activity in LIP and the latency of double-step performance. Panels show the distribution of r values for the trial-by-trial correlation between updating activity and latency of the second saccade. Conventions as in Fig. 9. For all trials combined (A,B) the distribution had a slight but nonsignificant positive skew for both split-brain and intact monkey, indicating that stronger updating activity was associated with longer latencies. This positive skew was significant in the intact monkey for within-hemifield trials alone (D). For all other cases in the split-brain (A,C,E) and intact monkey (B,F), there was no significant trial-by-trial relationship between updating activity and second-saccade latency on the double-step task.
Latency results for the intact monkey were virtually identical to those for the split-brain monkeys when the within and across trials were combined: the distribution of r values had a slight positive shift that approached significance (Fig 10B; mean r = .062, p= .09, sign test). In this combined analysis, a small proportion of single neurons showed significant trial-by-trial correlations (7/42, 17%), with most showing a positive relationship (5/7). We then conducted the trial-by-trial analysis separately for the two conditions. For the within condition, the distribution had a small but significant positive shift (Fig 10D; mean r = .105, p< .01, sign test), though the number of individual neurons with a significant correlation was very small (2/42, <1%, both positive). For the across condition, we found that the distribution of r values did not differ significantly from zero (Fig 10F; mean r = .056, p = .64, sign test).
In summary, these analyses indicate that updating activity in single LIP neurons is related to performance on the double-step task insofar as there are differences between within-hemifield and across-hemifield updating in the split-brain monkey. Independent of the conditional differences, however, we found no clear evidence that updating activity in single LIP neurons is correlated with behavioral performance on a trial-by-trial basis, in either the split-brain or the intact monkey.
Experience-dependent changes in behavior and neural activity
This extensive dataset gave us the opportunity to ask how double-step performance and neural activity in the split-brain monkey changed with experience. One of the most intriguing findings from our previous behavioral studies was that the split-brain monkeys exhibited initial impairments in performance on the across-hemifield condition but improved substantially as the animals gained experience with specific test sequences (Berman et al. 2005). In the present study, the spatial arrangement of double-step targets was determined by the location of the receptive field, and as a result, the monkeys were often presented with new double-step sequences. For one monkey, however, we had multiple testing sessions (n=35) in which the receptive field was placed at the same angle (45°) relative to central fixation (Fig. 11A). Consequently, the monkey had repeated experience with the same geometric arrangement of double-step targets. This allowed us to investigate the impact of experience on both the behavior and neural activity associated with spatial updating. We were interested in two questions. First, could we detect any experience-dependent change in the monkeys’ double-step performance on the across-hemifield and within-hemifield conditions? Second, if so, would these behavioral changes be accompanied by parallel changes in neural activity? We addressed these questions by looking at the Within:Across indices for the sessions in which the monkey performed the same configuration of the double-step task. These indices, described above, capture the relative bias toward within-hemifield updating (positive values) or across-hemifield updating (negative values). We reasoned that if experience improved the monkey’s performance on the across-hemifield as compared to the within-hemifield sequence, we would observe a shift in the behavioral Within:Across indices, with values becoming less positive.
Fig. 11.

Changes in behavior and neural activity over multiple sessions of testing with the same spatial geometry of the double-step saccade task. Each point represents data from a single recording session (one cell) in which the receptive field (RF) was located at the same angle, 45°, from fixation (A). In panels B–D, Within:Across indices (y axis) are plotted as a function of session (x axis); regression lines are indicated by the thin black lines. Positive index values denote greater accuracy (B), faster latency (C), or stronger updating activity (D) for the within-hemifield sequence. For accuracy and updating activity, index values approached zero over sessions, indicating that within and across updating became more alike as the monkey gained experience. For latency, index values became increasingly more negative, indicating that across-hemifield latencies became even shorter relative to within-hemifield latencies as the monkey gained experience.
We found that the monkey initially performed the across-hemifield sequence less accurately than the within-hemifield sequence: in the first few sessions, the values for the Within:Across accuracy index were +0.4 or greater (Fig. 11B), consistent with more accurate performance on the within-hemifield condition. With experience, however, there was a decreasing difference in accuracy for the across-hemifield as compared to the within-hemifield condition. This is evident in the index values, which became more negative over sessions (Fig. 11B). We found a significant correlation between the Within:Across accuracy index and session (r = −0.602, p < .001, Pearson’s), indicating that experience led to improved accuracy on the across-hemifield as compared to the within-hemifield double-step task. The effect of experience was even more apparent for saccade latency (Fig. 11C). In initial sessions with this specific double-step configuration, the monkey initiated the second saccade at about the same time for the across-hemifield and within-hemifield conditions (index values near zero). With experience, the monkey’s second saccade latencies became increasingly faster for the across-hemifield condition as compared to the within-hemifield condition (index values became more negative). The Within:Across latency index was significantly correlated with session (r = −0.679 p < .0001, Pearson’s). These accuracy and latency data show that experience was associated with improved behavioral performance on the across-hemifield condition as compared to the within-hemifield condition.
The second question we asked was whether we observed a similar experience-dependent change in neural activity. For example, does activity in area LIP neurons shift from being stronger for the within-hemifield condition (positive index values) to being more equivalent for the two conditions (index values near zero)? We found that neural activity indeed exhibited a trend that matched what we observed in the behavioral data: the Within:Across neural index became more negative over sessions and was significantly correlated with session (Fig. 11D; r = −0.482, p < .01, Pearson’s). Finally, we found a significant relationship between a single neuron’s Within:Across index and both of the behavioral Within:Across indices (neural index vs. accuracy index, r = .526, p < .01; neural index vs. latency index, r = .415, p < .05, Pearson’s). These results indicate that updating activity in area LIP changes in concert with behavior as the monkey gains experience with specific double-step sequences.
DISCUSSION
Our central hypothesis is that direct cortico-cortical links are integral to updating spatial representations when the eyes move. We have investigated this hypothesis in a series of experiments that assess the behavioral and physiological correlates of remapping in the split-brain monkey (Berman et al. 2005; Heiser et al. 2005). In the present study, we examined the split-brain monkeys’ performance on the double-step saccade task and the accompanying neural signals in parietal cortex. Three key observations emerge from this study. First, we found that split-brain monkeys continued to exhibit moderate impairment in the double-step task when across-hemifield updating was required, despite extensive training and experience with the task. This impairment was evident in increased spatial error on the across-hemifield condition. Second, we found that neural activity associated with across-hemifield updating in the double-step task was altered but by no means abolished in the absence of the forebrain commissures. In area LIP of split-brain monkeys, average updating activity was diminished and delayed in the across-hemifield condition but was nevertheless present in a majority of neurons. The double-step task elicited heightened activity compared to the single-step task, demonstrating an influence of behavioral relevance for both within-hemifield and across-hemifield conditions. Third, we found evidence that remapping in area LIP, when considered at the level of the population, corresponds to the accuracy of performance on the double-step task in the split-brain monkey. We discuss the implications of these new findings for the neural circuitry of spatial updating and the representation of space in area LIP.
The role of direct cortical links in spatial updating
Our present findings, together with our earlier behavioral and physiological studies, demonstrate that direct cortico-cortical links are an important part of the circuitry for spatial updating. In the absence of the forebrain commissures, spatial updating was less robust for double-step sequences that required the stimulus trace of the second target to be remapped across hemifields as compared to within the same hemifield. This was evident both in the monkeys’ performance of the double-step task and in the pattern of activity in area LIP. During these physiological recording sessions, the monkeys generated less accurate saccades in the across-hemifield condition as compared to the within-hemifield condition, despite many months of experience with both conditions of the task. Furthermore, single neuron activity in LIP was diminished in the across-hemifield condition, despite an increase in firing rate for the double-step as compared to the single-step task. These findings point toward the conclusion that direct cortico-cortical communication provides the most robust and rapid pathway for updating stimulus traces when the eyes move.
Subcortical pathways and recovered function
Our findings demonstrate that while direct cortico-cortical connections via the forebrain commissures are the primary pathway for remapping, they are not the only route by which remapped information can be transferred (Berman et al. 2005; Colby et al 2005; Heiser et al. 2005). Both split-brain monkeys were able to perform the across-hemifield double-step sequences, despite some inaccuracy, and showed no significant slowing of saccade latency. The majority of LIP neurons in the split-brain monkeys had significant updating activity in the across-hemifield condition of the double-step task. Furthermore, when we compared remapping for the double-step and single-step tasks, we found that the behavioral relevance of the to-be-updated stimulus was conveyed for both within-hemifield and across-hemifield conditions. These behavioral and neural data point toward an unexpected outcome of this series of experiments: spatial updating across visual hemifields is remarkably effective in the absence of the forebrain commissures.
What structures contribute to across-hemifield updating in the split-brain monkey? In the absence of the forebrain commissures, the SC may be a critical node for relaying remapped visual signals when across-hemifield updating is required. Neurons in the SC carry a range of visual, oculomotor, and cognitive signals, and can dynamically update visual representations in conjunction with eye movements (Munoz and Everling, 2004; Schiller and Stryker, 1972; Walker et al. 1995; Wurtz and Goldberg, 1971). In the normal monkey, the remapped visual signals observed in SC are thought to originate in cortex (Quaia et al. 1998). The frontal eye field sends remapped visual information to the SC (Sommer and Wurtz, 2003); area LIP is another likely source of these signals as it also projects directly to neurons in the intermediate layer of the SC (Ferraina et al. 2002; Pare and Wurtz, 1997, 2001). In addition to the SC, the pulvinar and cerebellum have been implicated as important pathways for interhemispheric transfer in the absence of the forebrain commissures (Corballis, 1995; Glickstein, 1990). Furthermore, there is considerable interest in the proposal that thalamic nuclei may serve to mediate cortico-cortical communication (Guillery and Sherman 2002). These findings underscore the likelihood that a broad network of regions work together to carry out across-hemifield updating when the predominant pathways – the forebrain commissures – are absent.
The resilience of across-hemifield updating in the split-brain monkey is reminiscent of previous demonstrations of recovery of function in the oculomotor system. Lesions of the superior colliculus or frontal eye field induce deficits in saccadic eye movements, but these deficits are transient unless both structures are removed (Schiller et al. 1979). If either the SC or FEF remains intact, voluntary saccade generation recovers spontaneously within several weeks. These findings have been interpreted as evidence of parallel pathways (Schiller et al. 1980), and more recently as evidence of reorganization in the oculomotor system (Hanes and Wurtz, 2001). Our results show that spatial updating is likewise subserved by a flexible cortical and subcortical network. Plasticity is not limited to the circuit for generating eye movements but is also present in the circuit responsible for the perceptual consequences of eye movements. These findings highlight the importance of the neural systems that use motor signals to modify sensory representations in the primate brain.
In keeping with these findings, we recently reported that the communication of corollary discharge information does not rely on direct cortico-cortical links (Colby et al. 2005). Corollary discharge, a copy of the motor command to move the eyes, is presumed to initiate the updating of visual representations (Duhamel et al. 1992a; Quaia et al. 1998), and recent experiments provide direct evidence that this is the case (Sommer and Wurtz 2006). Our observations indicate that corollary discharge signals, presumably originating in one hemisphere, can initiate spatial updating in the opposite hemisphere. This conclusion is consistent with recent evidence that subcortical structures play an important role in conveying corollary discharge information to cortex (Bellebaum et al. 2005; Crapse and Sommer, 2006; Sommer and Wurtz, 2002, 2006). Further delineation of these pathways will be essential to understanding both the behavioral and physiological correlates of remapping.
Modulation of neural activity in area LIP by behavioral relevance
One of the most striking features of LIP activity in the double-step saccade task is that firing rates are increased relative to the single-step task (Goldberg et al. 1990). This increase presumably reflects the added contributions of both oculomotor and attentional factors. The oculomotor contribution follows from the observation that many neurons in LIP have saccade-related activity, as measured in the memory-guided saccade task (Barash et al. 1991; Colby et al. 1996). In the double-step task, the second saccade is directed into the neuron’s response field, eliciting activity that could add to the remapped response. These eye movement signals alone, however, cannot account for increased responsivity in the double-step task; neurons are active in the double-step task even when they have no saccade-related activity (Goldberg and Bruce 1990; Goldberg et al. 1990; Walker et al. 1995). Attention also contributes to the intensified activity in the double-step task. Remapping in LIP is modulated by the salience of the stimulus to be updated (Gottlieb et al. 1998). This attentional modulation is consistent with multiple lines of evidence that suggest a role for area LIP in encoding salient visual stimuli (Assad 2003; Bisley and Goldberg 2003; Wardak et al. 2004). Together, these findings indicate that the heightened neural activity in the double-step task represents a concatenation of visual, oculomotor, and attentional signals.
In the split-brain monkey, we observed heightened activity in the double-step task both for updating within hemifields and updating across hemifields. Without direct cortical links, it seemed possible that the system would be less effective in relaying the behavioral relevance of the to-be-updated stimulus from one hemisphere to the other (Hines et al. 2002; Reuter-Lorenz and Fendrich 1990). We found, however, that the behavioral relevance was conveyed well for both within-hemifield and across-hemifield updating. This finding is consistent with earlier studies in split-brain patients, which indicate that subcortical structures can mediate the interhemispheric communication of attentional signals (Corballis 1995).
Area LIP and spatial behavior
Parietal cortex has long been known to be essential to the performance of spatial tasks (Andersen 1987; Bisiach and Luzzatti 1978; Colby and Goldberg 1999; Li and Andersen 2001; Paterson and Zangwill 1944). More recently, physiological studies have begun to ask how neural activity within parietal cortex relates to behavior. A number of studies have focused on decision processes, and suggest that activity in area LIP can predict perceptual choice or the monkey’s preference among competing actions (Dorris and Glimcher 2004; Platt and Glimcher 1999; Shadlen and Newsome 2001; Sugrue et al. 2004; Williams et al. 2003). Recent studies have investigated whether single neuron activity in LIP relates in any systematic way to the generation of saccadic eye movements. For example, several experiments have shown a significant correlation between saccade latencies and the time of saccade selection signals in LIP in a visual search task (Ipata et al. 2006, Thomas and Pare 2007). Our experiment differs from these recent studies in two important ways. First, we have focused on a specific aspect of LIP function – updating activity – and have asked if it is related to the monkeys’ ability to perform the second saccade in the double-step task. Second, our investigation focused not on the normal animal, but on split-brain monkeys. In the present study, we capitalized on the variability in LIP updating activity and behavioral performance in the split-brain monkeys, and asked whether the two are related.
Our results indicate a parallel between the strength of updating activity in LIP neurons and the accuracy of double-step performance in the split-brain monkey. In single neurons, stronger updating activity in LIP was associated with more accurate performance of the second saccade when within and across trials were analyzed together. When within and across trials were analyzed separately, however, we found no trial-by-trial relationship between updating activity and accuracy. Rather, the relationship in single cells reflects the conditional differences between within-hemifield and across-hemifield updating. In our population analysis, we focused on the relative difference between within-hemifield and across-hemifield updating. Average updating activity in area LIP was stronger for within-hemifield than across-hemifield updating, and, in parallel, the monkeys were more accurate for within-hemifield than across-hemifield updating. These findings imply that the spatial coordinates for the second saccade in the double-step task may be extracted from the remapped responses of an ensemble of LIP neurons. This is compatible with the suggestion that population activity in LIP can guide the direction of saccades generated in the antisaccade task (Zhang and Barash, 2000), and is consistent with other findings of population coding in the oculomotor system (Lee et al. 1988).
We observed an unanticipated relationship between updating activity and second-saccade latency in the split-brain monkeys: stronger updating activity was associated with prolonged saccade latencies instead of faster latencies as might be expected. At the level of single neurons, we found that some cells had significant, positive trial-by-trial correlations between updating activity and latency when within- and across-hemifield trials were analyzed together. At the level of the population, updating activity was biased toward stronger firing in the within-hemifield condition, while latencies were faster on average for the across-hemifield sequences. This apparent dissociation between remapping signals in LIP and latency is intriguing, given the unusually short second-saccade latencies for across-hemifield sequences. The short latencies emerged with experience and indicate that the split-brain monkeys may have established a more automated strategy to generate the across-hemifield sequences. It would be useful to determine whether updating activity in other structures, such as the FEF or SC, might be more closely linked to second-saccade latency in the double-step task. Further investigation of these structures will be important to determine the compensatory pathways that contribute to double-step performance in the absence of the forebrain commissures.
In the intact monkey, there was a parallel between updating activity and behavior when we considered the overall population bias toward either within-hemifield or across-hemifield updating. This population analysis showed no relative differences between within-hemifield and across-hemifield conditions for any of the three measures –updating activity, saccade accuracy, or saccade latency. In our single neuron analysis, we did not observe strong evidence for a significant trial-by-trial correlation between updating activity and either accuracy or latency of the second saccade. This may be because performance was consistently good. In one of the seminal studies that uncovered a significant relationship between the activity of single neurons and behavior, an important feature was that the monkeys’ performance was at threshold, with a number of successful as well as unsuccessful trials (Newsome et al. 1989). In the intact monkey, there was little variability in performance of the double-step task, which may have impeded detection of clear trial-by-trial correlations between updating activity and behavior.
It is important to note that, insofar as there is a correspondence between updating activity and double-step performance in the split-brain monkey, we cannot address the possibility of a causal relationship between these variables. For example, inaccurate double-step performance on the across-hemifield condition may be the result of reduced updating activity in area LIP. Alternatively, inaccurate performance may be due to disrupted activity in other structures, and the inaccurate performance itself could cause reduced activity in LIP. The present experiment was not designed to distinguish between these two possibilities. We can conclude, however, that there are parallels in the split-brain monkey between remapping in area LIP as a whole, and the overall performance of the double-step task.
In conclusion, these experiments have yielded three new findings about the dynamic nature of the circuitry for spatial updating in the primate brain. First, in the absence of direct cortico-cortical communication, animals are able to perform the double-step task well for targets whose representation has to be remapped across hemispheres, in addition to those whose representation remains within one hemisphere. Performance is more accurate for the within-hemifield than the across-hemifield condition, despite the split-brain monkeys’ extensive experience with the task. Second, in parallel to the behavioral results, LIP population activity in the split-brain monkey is stronger for within-hemifield as compared to across-hemifield updating. Third, the difference in accuracy between within-hemifield and across-hemifield performance corresponds to differences in updating activity; this correspondence is detectable in single neurons in area LIP. This finding emphasizes that parietal cortex is an integral part of the network that updates spatial representations for the accurate guidance of eye movements. These experiments highlight the importance of direct cortico-cortical links for spatial updating, yet also reveal considerable plasticity in the circuitry that constructs an internal representation of a stable visual world.
Acknowledgments
We thank K. McCracken and Dr. Kevin Hitchens for technical assistance, and our colleagues at the Center for the Neural Basis of Cognition for constructive comments. Present addresses: R. Berman, Laboratory of Sensorimotor Research, National Eye Institute, National Institutes of Health, Bethesda MD 20892; L. Heiser, Life Sciences Division, Lawrence Berkeley National Lab, Berkeley, CA 94720.
Grants This work was supported by National Institutes of Health Grants EY-12032 and MH-45156, technical support was provided by core grant EY-08908, and collection of MR images was supported by P41RR-03631. Support was also provided by National Science Foundation Fellowship to R.A. Berman, and National Aeronautics and Space Administration Fellowships to C.A. Dunn and L.M. Heiser.
References
- Andersen RA. Handbook of Physiology: The Nervous System. Bethesda, MD: Williams Wekens; 1987. Inferior parietal lobule function in spatial perception and visuomotor integration; pp. 483–518. [Google Scholar]
- Assad JA. Neural coding of behavioral relevance in parietal cortex. Curr Opin Neurobiol. 2003;13:194–197. doi: 10.1016/s0959-4388(03)00045-x. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Bender DB. Comparison of saccadic eye movements in humans and macaques to single-step and double-step target movements. Vision Res. 1989;29:485–495. doi: 10.1016/0042-6989(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Baker JT, Harper TM, Snyder LH. Spatial memory following shifts of gaze. I. Saccades to memorized world-fixed and gaze-fixed targets. J Neurophysiol. 2003;89:2564–2576. doi: 10.1152/jn.00610.2002. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol. 1991;66:1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- Berman RA, Heiser LM, Saunders RC, Colby CL. Dynamic circuitry for updating spatial representations. I. Behavioral evidence for interhemispheric transfer in the split-brain macaque. J Neurophysiol. 2005;94:3228–3248. doi: 10.1152/jn.00028.2005. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14 doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Colby CL, Berman RA, Heiser LM, Saunders RC. Corollary discharge and spatial updating: when the brain is split, is space still unified? Prog Brain Res. 2005;149:187–205. doi: 10.1016/S0079-6123(05)49014-7. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Visual integration in the split brain. Neuropsychologia. 1995;33:937–959. doi: 10.1016/0028-3932(95)00032-x. [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Crossed connections in the visuosaccadic system: Frontal eye field neurons orthodromically activated from the contralateral superior colliculus. Soc Neurosci Abstr. 2006:439.13. [Google Scholar]
- Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 2004;44:365–378. doi: 10.1016/j.neuron.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992a;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Goldberg ME, Fitzgibbon EJ, Sirigu A, Grafman J. Saccadic dysmetria in a patient with a right frontoparietal lesion. The importance of corollary discharge for accurate spatial behaviour. Brain. 1992b;115:1387–1402. doi: 10.1093/brain/115.5.1387. [DOI] [PubMed] [Google Scholar]
- Ferraina S, Pare M, Wurtz RH. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol. 2002;87:845–858. doi: 10.1152/jn.00317.2001. [DOI] [PubMed] [Google Scholar]
- Glickstein ME. Brain pathways in the visual guidance of movement and the behavior functions of the cerebellum. In: Trevarthen C, editor. Brain circuits and function of the mind: essays in honor of R. W. Sperry. Cambridge: Cambridge University Press; 1990. pp. 158–167. [Google Scholar]
- Goldberg ME, Bruce CJ. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. J Neurophysiol. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Colby CL, Duhamel JR. Representation of visuomotor space in the parietal lobe of the monkey. Cold Spring Harb Symp Quant Biol. 1990;55:729–739. doi: 10.1101/sqb.1990.055.01.068. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res. 1976;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol. 2001;85:804–815. doi: 10.1152/jn.2001.85.2.804. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Colby CL. Spatial Updating in Area LIP is Independent of Saccade Direction. J Neurophysiol. 2006;95:2751–2767. doi: 10.1152/jn.00054.2005. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Berman RA, Saunders RC, Colby CL. Dynamic circuitry for updating spatial representations. II. Physiological evidence for interhemispheric transfer in area LIP of the split-brain macaque. J Neurophysiol. 2005;94:3249–3258. doi: 10.1152/jn.00029.2005. [DOI] [PubMed] [Google Scholar]
- Hines RJ, Paul LK, Brown WS. Spatial attention in agenesis of the corpus callosum: shifting attention between visual fields. Neuropsychologia. 2002;40:1804–1814. doi: 10.1016/s0028-3932(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332:357–369. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Li CS, Andersen RA. Inactivation of macaque lateral intraparietal area delays initiation of the second saccade predominantly from contralesional eye positions in a double-saccade task. Exp Brain Res. 2001;137:45–57. doi: 10.1007/s002210000546. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol. 1980;43:207–232. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Motor intention activity in the macaque lateral intraparietal area. I. Dissociation of motor plan from sensory memory. J Neurophysiol. 1996;76:1439–1456. doi: 10.1152/jn.1996.76.3.1439. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson K. Frontal eye field contributions to rapid corrective saccades. J Neurophysiol. 2007;97:1457–1469. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Visual, saccade-related, and cognitive activation of single neurons in monkey extrastriate area V3A. J Neurophysiol. 2000;84:677–692. doi: 10.1152/jn.2000.84.2.677. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci U S A. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Pare M, Wurtz RH. Monkey posterior parietal cortex neurons antidromically activated from superior colliculus. J Neurophysiol. 1997;78:3493–3497. doi: 10.1152/jn.1997.78.6.3493. [DOI] [PubMed] [Google Scholar]
- Pare M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area LIP to superior colliculus. J Neurophysiol. 2001;85:2545–2562. doi: 10.1152/jn.2001.85.6.2545. [DOI] [PubMed] [Google Scholar]
- Paterson A, Zangwill O. Disorders of visual space perception associated with lesions of the right cerebral hemispheres. Brain. 1944:67. [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Quaia C, Optican LM, Goldberg ME. The maintenance of spatial accuracy by the perisaccadic remapping of visual receptive fields. Neural Netw. 1998;11:1229–1240. doi: 10.1016/s0893-6080(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Ray S, Schall JD, Murthy A. Programming of double-step saccade sequences: modulation by cognitive control. Vision Res. 2004;44:2707–2718. doi: 10.1016/j.visres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Fendrich R. Orienting attention across the vertical meridian: Evidence from callosotomy patients. J Cog Neurosci. 1990;2:232–238. doi: 10.1162/jocn.1990.2.3.232. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Effects of frontal eye field and superior colliculus ablations on eye movements. Science. 1979;206:590–592. doi: 10.1126/science.115091. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol. 1980;44:1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker MP. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972;35:915–924. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. The frontal eye field sends predictively remapped visual signals to the colliculus. J Vision. 2003;3:146a. [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci. 2004;24:9291–9302. doi: 10.1523/JNEUROSCI.2671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Saunders RC, Orban GA. Hemispheric lateralization in rhesus monkeys can be task-dependent. Neuropsychologia. 1994;32:425–438. doi: 10.1016/0028-3932(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Walker MF, Fitzgibbon EJ, Goldberg ME. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. J Neurophysiol. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA. Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci. 2003;6:616–623. doi: 10.1038/nn1055. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science. 1971;171:82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408:971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]