Abstract
Olfactory bulb glomeruli are formed by a network of three major types of neurons collectively called juxtaglomerular (JG) cells, which include external tufted (ET), periglomerular (PG), and short axon (SA) cells. There is solid evidence that γ-aminobutyric acid (GABA) released from PG neurons presynaptically inhibits glutamate release from olfactory nerve terminals via activation of GABAB receptors (GABAB-Rs). However, it is still unclear whether ET cells have GABAB-Rs. We have investigated whether ET cells have functional postsynaptic GABAB-Rs using extracellular and whole cell recordings in olfactory bulb slices. In the presence of fast synaptic blockers (CNQX, APV, and gabazine), the GABAB-R agonist baclofen either completely inhibited the bursting or reduced the bursting frequency and increased the burst duration and the number of spikes/burst in ET cells. In the presence of fast synaptic blockers and tetrodotoxin, baclofen induced an outward current in ET cells, suggesting a direct postsynaptic effect. Baclofen reduced the frequency and amplitude of spontaneous EPSCs in PG and SA cells. In the presence of sodium and potassium channel blockers, baclofen reduced the frequency of miniature EPSCs, which were inhibited by the calcium channel blocker cadmium. All baclofen effects were reversed by application of the GABAB-R antagonist CGP55845. We suggest that activation of GABAB-Rs directly inhibits ET cell bursting and decreases excitatory dendrodendritic transmission from ET to PG and SA cells. Thus the postsynaptic GABAB-Rs on ET cells may play an important role in shaping the activation pattern of the glomeruli during olfactory coding.
INTRODUCTION
γ-Aminobutyric acid type B receptors (GABAB-Rs) are broadly expressed in the nervous system and the GABAB-R agonist baclofen is used clinically to treat a wide variety of neurological and psychiatric disorders (for review, see Bettler et al. 2004; Bowery 2006). In the rat main olfactory bulb, the glomeruli have the highest concentration of GABAB-Rs as determined by radioligand binding (Bowery et al. 1987; Chu et al. 1990) and by immunohistochemical localization of GABAB-R subunits (Bonino et al. 1999; Margeta-Mitrovic et al. 1999). There is now strong evidence for GABAB-R–mediated presynaptic inhibition of glutamate release from olfactory nerve terminals (Aroniadou-Anderjaska et al. 2000; Keller et al. 1998; Murphy et al. 2005; Nickell et al. 1994; Palouzier-Paulignan et al. 2002; Vucinic et al. 2006; Wachowiak and Cohen 1999; Wachowiak et al. 2005). However, it is unknown whether GABAB-Rs are located postsynaptically on juxtaglomerular (JG) cells and whether they can modulate the bursting pattern of external tufted (ET) cells.
GABAB-Rs are heterodimers consisting of two distinct subunits: GABAB1 and GABAB2 (for review, see Bettler et al. 2004; Emson 2007). There have been few studies suggesting the presence of GABAB-R subunits on unidentified JG neurons. Immunolabeling for GABAB2-Rs was found at symmetric dendrodendritic synapses and was associated with perisynaptic sites and the cytoplasmic face of the postsynaptic membrane, suggesting that postsynaptic GABAB-Rs may be expressed at junctions between periglomerular (PG) interneurons and projection neurons (Kratskin et al. 2006). Bonino et al. (1999) have shown the presence of postsynaptic GABAB-Rs on JG neurons using an antibody for both splice variants of the receptors (GABAB1a and GABAB1b). It is unknown whether the GABAB1 and GABAB2 subunits are colocalized at glomerular synapses to form functional GABAB-Rs.
In the hippocampus, GABAB-R activation has been reported to have an anticonvulsant-like effect by reducing the frequency of epileptiform burst firing (Ault et al. 1986; Swartzwelder et al. 1986). Usually metabotropic receptors are activated during bursts of synaptic input, during which a relatively high concentration of neurotransmitter is released in the synaptic cleft. In the olfactory bulb, ET cells exhibit rhythmic, intrinsically generated spike bursts at theta frequencies, as reported both in vivo (Getchell and Shepherd 1975; Wellis and Scott 1990) and in vitro (Antal et al. 2006; Hayar et al. 2004b). They receive monosynaptic olfactory nerve input and provide intraglomerular excitatory monosynaptic input to PG and short axon (SA) cells (Hayar et al. 2004a). PG cells release GABA in a synchronized bursting fashion onto ET cells to induce feedback inhibition (Hayar et al. 2005). One consequence of this GABA release might be to activate postsynaptic GABAB-Rs on ET cells and modulate their spontaneous bursting.
The effects of baclofen on the mode of bursting and on the membrane currents of ET cells might lead to modulation of dendrodendritic synaptic transmission between ET and PG cells. In particular, GABAB-Rs may modulate calcium channels, which were recently shown to be located mostly on the distal dendrites of ET cells (Zhou et al. 2006). Activation of presynaptic GABAB-Rs reduces neurotransmitter release at many central synapses (Chen and Pan 2006; Misgeld et al. 1995). For instance, baclofen decreases GABA and glutamate release in hypothalamic paraventricular neurons (Cui et al. 2000; Wang et al. 2003). However, in CA3 neurons of the hippocampus, baclofen decreases the frequency of miniature excitatory postsynaptic currents (mEPSCs), but not the frequency of miniature inhibitory postsynaptic currents (mIPSCs) (Scanziani et al. 1992). These studies suggest that the synaptic GABA and glutamate release may be differentially regulated by GABAB-Rs in different brain regions. JG cells communicate mainly by dendrodendritic synapses (Pinching and Powell 1971a,b). Therefore it would be interesting to test whether GABAB-Rs could modulate neurotransmitter release from dendrites in the same fashion as in axon terminals in other brain regions.
In the olfactory bulb, the presynaptic inhibition by GABAB-Rs may serve as a means of fine-tuning synaptic strength or as negative feedback to prevent excessive transmitter release and to reduce the overall energy demands of the glomeruli (Nawroth et al. 2006). In contrast, it is unknown whether there are functional postsynaptic GABAB-Rs that limit the range of excitation of ET cells and modulate their dendrodendritic glutamate release onto other JG neurons. We hypothesize that both the presynaptic inhibition of olfactory nerve terminals and the postsynaptic GABAB-R–mediated inhibitory effect of ET cells are important for shaping the activation pattern of the glomeruli during olfactory coding. In this study, we test whether activation of GABAB-Rs directly inhibits ET cell bursting, leading to a reduction of excitatory drive to PG and SA cells.
METHODS
Slice preparation
Sprague–Dawley rats (21–29 days old), of either sex, were decapitated in accordance with Institutional Animal Care and Use Committee and National Institutes of Health guidelines. The olfactory bulbs were removed and immersed in sucrose–artificial cerebrospinal fluid 7.38). The (sucrose-ACSF) equilibrated with 95% O2-5% CO2 (pH = 7.38). The sucrose-ACSF had the following composition (in mM): 26 NaHCO3, 3 KCl, 8 MgCl2, 0.5 CaCl2, 20 glucose, 0.4 ascorbic acid, 2 sodium pyruvate, and 234 sucrose. Horizontal slices (400 μm thick) were cut with a microslicer (Ted Pella, Redding, CA). After a period of recovery at 30°C for 15 min, slices were then incubated at room temperature (22°C) in ACSF equilibrated with 95% O2-5% CO2 [composition (in mM): 124 NaCl, 26 NaHCO3, 4 KCl, 2 MgCl2, 2 CaCl2, 0.4 ascorbic acid, 2 sodium pyruvate, and 20 glucose], until studied at 30°C. For recording, a single slice was placed in a recording chamber and continuously perfused at the rate of 1–1.5 ml/min with normal ACSF equilibrated with 95% O2-5% CO2.
Electrophysiological recordings
All recordings were performed at 30°C. Neurons were visualized using an upright microscope (BX50WI, Olympus, Tokyo, Japan) equipped with epifluorescence. The image of the neurons was displayed on a 17-in. LCD flat panel TV (Samsung SyncMaster 710MP, resolution 1,280 × 1,024 pixels). The S-video input of the monitor was connected to the S-video output of a 3-CCD color camera (Hitachi HV-D30) that was installed on the microscope via a C-mount adapter. Extracellular recordings (loose patch) were obtained using pipettes (resistance was 6–10 MΩ) filled with ACSF. Extracellular spikes were recorded using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) as action currents in voltage-clamp mode at a holding potential (HP) of 0 mV and using a high-pass filter of 0.3–1 Hz. The advantages of using this recording configuration have been discussed recently (Perkins 2006). For patch-clamp recordings of ET cells, electrodes were filled with a solution of the following composition (in mM): 124 K-gluconate, 10 phosphocreatine di(tris) salt, 10 HEPES, 10 BAPTA, 4 Mg2ATP, 0.3 Na2GTP, and 0.383 mM Lucifer Yellow (pH 7.3 adjusted with KOH, osmolarity 266 mOsm). For patch-clamp recordings of PG and SA cells, electrodes were filled with a solution of the following composition (in mM): 125 CsMeSO3, 10 phosphocreatine di(tris) salt, 10 HEPES, 10 BAPTA, 10 QX-314, 4 Mg2ATP, 0.3 Na2GTP, and 0.383 mM Lucifer Yellow (pH 7.3 adjusted with NaOH, osmolarity 284 mOsm). Pipettes were pulled from borosilicate glass capillaries with an inner filament (1.5 mm OD, Clark, Kent, UK) on a pipette puller (P-97, Sutter Instrument, Novato, CA). Patch electrodes (resistance 8–12 MΩ) for whole cell recordings were pulled using the same parameters as those used for loose-patch extracellular recordings. Electrical stimulation was performed via a Master-8cp stimulator (AMPI, Jerusalem, Israel) using two stainless steel wires (50 μm in diameter; A–M Systems, Everett, WA), insulated except at their tips. Isolated, constant-current stimulus pulses of 20–200 μA and 200-μs duration were applied using an ISO-Flex stimulus isolation unit (AMPI).
Data analysis
Analog signals were low-pass filtered at 2 kHz (Multiclamp 700B) and digitized at 5 kHz using a Digidata-1322A interface and pClamp-9 software (Molecular Devices). Spike detection and burst parameter calculations were performed off-line using custom-designed software. Data were then imported into Origin 7.0 (Microcal Software, Northampton, MA) for further analysis using algorithms written in LabTalk. We evaluated the pattern of bursting of spikes throughout the experiments by measuring the following parameters: overall firing frequency, interburst frequency (defined as the number of bursts/s), number of spikes/burst, intraburst frequency (defined as the frequency of spikes within a burst), and burst duration (defined as the time interval between the first and the last spikes in a burst). The intraburst interspike interval (ISI) slope (ms/interval) was calculated using the linear regression fit of the ISI within the burst versus the interval number. The slope was determined by the linear regression formula: slope = Σ {[X(n) − Xm] × [Y(n) − Ym]}/Σ [X(n) − Xm]2, where n is the index number, X(n) is the interval number, Y(n) is the interval duration, and Xm and Ym are mean values of the interval number and interval duration, respectively (Karpuk and Vorobyov 2003). A burst of spikes was defined as a series of three or more consecutive spikes that had interspike time intervals of <75 ms (i.e., an interspike time interval of >75 ms was considered to signal the beginning of another burst). Autocorrelograms (Fig. 2) were normalized by dividing the counts in each bin by a coefficient (N), which is the number of events expected by chance during one bin: N = F1 × F2 × P × B; where F1 is the mean intraburst frequency (Hz), F2 is the mean firing frequency (Hz), P is the sampling period (s) used for analysis, and B is the duration (s) of one bin. Therefore the coefficient of autocorrelation (C) at a different time lag indicates the probability of occurrence of spikes in one bin compared with chance (i.e., when we assume random occurrence of spikes). Data, expressed as means ± SE, were statistically analyzed using two-tailed paired t-test (Microsoft Excel 2003) unless otherwise stated.
Drugs
Drugs were applied to the bath solution of slices by switching the perfusion with a three-way valve system. Cesium methanesulfonate (CsMeSO3), tetraethylammonium chloride (TEA), cadmium chloride (CdCl2), barium chloride dihydrate (BaCl2), L-ascorbic acid, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (±)-2-amino-5- phosphopentanoic acid (APV), lidocaine N-ethyl bromide (QX-314), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrapotassium salt (BAPTA), and phosphocreatine di(tris) salt were purchased from Sigma–Aldrich (St. Louis, MO). Gabazine (SR95531), 3-N-[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid (CGP55845), (RS)-baclofen, and tetrodotoxin (TTX) were purchased from Tocris Bioscience (Ellisville, MO).
RESULTS
Baclofen modulates spontaneous bursting of ET cells
We first investigated the effects of baclofen on ET cells using the loose-patch technique. The advantage of this recording configuration is that the activity of ET cells is stable and does not undergo rundown as in whole cell recordings due to intracellular dialysis (Hayar et al. 2004b). To assess the postsynaptic effect of baclofen, we recorded ET cells in the presence of blockers of fast synaptic transmission, which included the non–N-methyl-D-aspartate (NMDA) receptor antagonist CNQX (10 μM), the NMDA receptor antagonist APV (50 μM), and the GABAA receptor antagonist gabazine (10 μM), which are referred to here as fast synaptic blockers. Under these conditions, any effect observed would most likely be attributable to direct activation of GABAB-Rs located on ET cells since the major synaptic inputs to ET cells have been blocked (Hayar and Ennis 2007; Hayar et al. 2004a,b, 2005). ET cells can be readily identified in extracellular recordings because they exhibit spontaneous bursting that persists in the presence of synaptic blockers (Hayar and Ennis 2007; Hayar et al. 2004a,b, 2005). Other cells that stopped bursting during drug application were excluded from the analysis. The bursting properties of the cells during application of the fast synaptic blockers were taken as control.
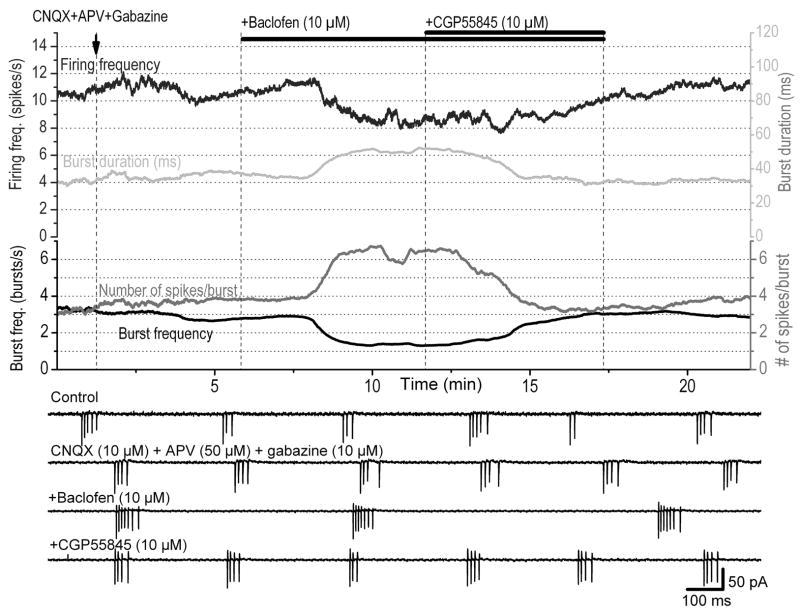
In a small percentage of ET cells (3 of 13 cells), baclofen (10 μM) completely inhibited the firing activity of ET cells. However, in most cases (10 of 13 cells), the effects of baclofen could not be explained simply by inhibition of firing activity. To analyze in detail the effects of baclofen, we examined the pattern of bursting activity throughout the experiment by measuring the following parameters: firing frequency, interburst frequency, intraburst frequency, number of spikes/burst, burst duration, and intraburst ISI slope (see methods for definitions). A moving average of each parameter was computed to dampen fast variations. Typical responses of baclofen on the bursting properties of an ET cell recorded extracellularly are shown in Fig. 1 and a statistical analysis on a group of 10 cells is shown in Table 1. Although not all cells were affected to the same degree by baclofen, on average, all bursting parameters showed statistically significant changes during baclofen application. All baclofen-induced effects almost completely reversed after addition of the GABAB-R blocker CGP55845 (10 μM).
FIG. 1.
Effects of baclofen on spontaneous spike bursting in external tufted (ET) cells. Extracellular recordings were made from an ET cell exhibiting bursts of actions potentials that persisted in the presence of blockers of fast synaptic transmission [6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 10 μM; (±)-2-amino-5- phosphopentanoic acid (APV), 50 μM; gabazine, 10 μM]. The burst parameters are presented by different colors in the graphs with corresponding scales. Baclofen reduced the frequency of bursts and the firing frequency but it increased the burst duration and the number of spikes/burst. These effects were reversed after addition of the μ -aminobutyric type B receptor (GABAB-R) blocker CGP55845 to the bath (shown by the double bars above). The traces were smoothed by adjacent averaging from 30 consecutive data points. The timescale is common for all graphs. Bottom: 4 representative traces of extracellular recordings in each condition.
TABLE 1.
Effects of baclofen on the bursting properties of ET cells
| CNQX + APV + Gabazine | Baclofen | CGP55845 | |
|---|---|---|---|
| Spike frequency, Hz | 9.7 ± 1.7 | 6.9 ± 1.6** | 9.2 ± 1.5** |
| Intraburst frequency,Hz | 88.0 ± 9.5 | 103.0 ± 10.0** | 81.5 ± 10.0** |
| Burst frequency, Hz | 2.5 ± 0.5 | 1.8 ± 0.5*** | 2.2 ± 0.5** |
| Burst duration, ms | 42.0 ± 6.0 | 47.0 ± 6.0* | 44.0 ± 6.0 |
| Number of spikes/burst | 4.6 ± 0.4 | 5.7 ± 0.5** | 4.6 ± 0.4** |
| Burst interspike interval slope, ms/interval | 5.6 ± 1.2 | 3.4 ± 0.84** | 5.9 ± 1.4** |
Values are means ± SE; n = 10. Values in each column were statistically compared to the corresponding values in the previous column:
P < 0.05,
P < 0.01,
P < 0.001.
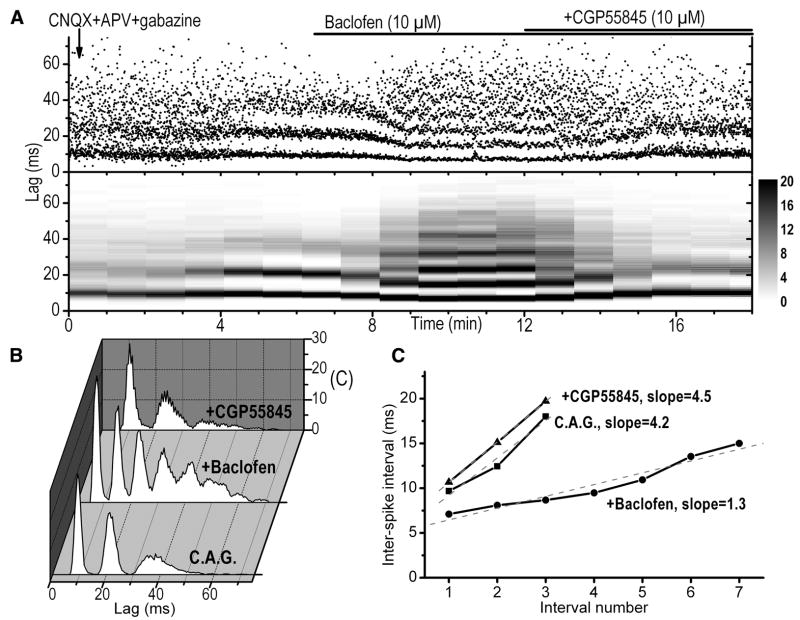
Baclofen exerted a prominent effect on the spike, interburst, and intraburst frequencies. It reduced the spike frequency from 9.7 ± 1.7 to 6.9 ± 1.6 Hz (P < 0.01) and the interburst frequency from 2.5 ± 0.5 to 1.8 ± 0.5 bursts/s (P < 0.001). In contrast, baclofen significantly increased the number of spikes per burst from 4.6 ± 0.4 to 5.7 ± 0.5 spikes/bursts (P < 0.01, Fig. 1) and the burst duration from 42 ± 6 to 47 ± 6 ms (P < 0.05). The timing parameters of the bursts were further analyzed by constructing the autocorrelograms of intraburst intervals (Fig. 2, A and B). There was a progressive increase in ISI within individual bursts. We quantified this property by calculating the intraburst ISI slope using the linear regression method (Fig. 2C). Under control conditions, this slope was 5.6 ms/interval, on average, indicating that every consecutive spike within a burst occurred with an interval that is about 5.6 ms longer than the previous ISI. The intraburst ISI slope was decreased by baclofen from 5.6 ± 1.2 to 3.4 ± 0.84 ms/interval (P < 0.01), suggesting that there is a tendency of relatively less spike firing adaptation within the burst during activation of the GABAB-Rs. The average intraburst spike frequency, which takes into account all ISIs within a burst, increased from 88 ± 9.5 to 103 ± 10 Hz (P < 0.01). Taken together, these results suggest that although the firing and interburst frequencies of ET cells were reduced by baclofen, the bursts become stronger since there were more spikes in a burst and these spikes occurred at shorter intervals.
FIG. 2.
Modulation of intraburst properties by baclofen. A, top: scatter plot of the interspike intervals (ISIs) calculated from the beginning of first spike in the burst under different pharmacological conditions. Bottom: sliding color-coded autocorrelograms (triggered on the first spike in the burst) performed every minute using 2-min sampling periods. Application of 10 μM CNQX, 50 μM APV, and 10 μM gabazine increased the regularity of ISIs within the burst as shown by the decrease in the variability of the data points that show higher density around regularly spaced peaks. Additional application of baclofen reduced the ISIs and introduced additional peaks due to the recruitment of more spikes. This effect was reversed by the GABAB-R blocker CGP55845. B: autocorrelograms of ISIs within bursts constructed using spike occurrence in 2-min sampling periods under different pharmacological conditions. The autocorrelograms were normalized to the bin width, the sampling period, the firing frequency, and the interburst frequency (see METHODS). C: evaluation of the slopes of the ISIs as a function of the interval number within a burst. The slopes were calculated using linear regression fit under different pharmacological conditions. All data obtained in this figure were obtained from the same cell.
Despite the effectiveness of CGP55845 in reversing the effects of baclofen on the bursting pattern (Table 1), we did not observe any significant effect of CGP55845 when applied alone. For instance, the spike firing rate under control conditions was not significantly changed by application of CGP55845 (control: 8.9 ± 2.3 Hz; CGP55845: 9.8 ± 2.9 Hz, n = 7, P = 0.24). Similarly, the interburst frequency was not significantly changed by application of CGP55845 (control: 2.5 ± 0.7 bursts/s; CGP55845: 2.6 ± 0.76 bursts/s, n = 5, P = 0.24). These results suggest that the bursting pattern of ET cells can be modulated by activation of GABAB-Rs, although these receptors do not seem to be tonically active under our testing conditions. It is possible that GABAB-Rs might be activated during olfactory nerve stimulation, which triggers massive GABA release from PG cells leading to a barrage of IPSCs in ET cells (Hayar et al. 2005). However, endogenous GABA, released by olfactory nerve stimulation, also activates presynaptic GABAB-Rs located on the olfactory nerve terminals (Aroniadou-Anderjaska et al. 2000). Therefore it is not technically possible to differentiate between activation of the pre-versus postsynaptic GABAB-Rs because of the lack of selective antagonists.
Baclofen exerts direct effects on the membrane properties of ET cells
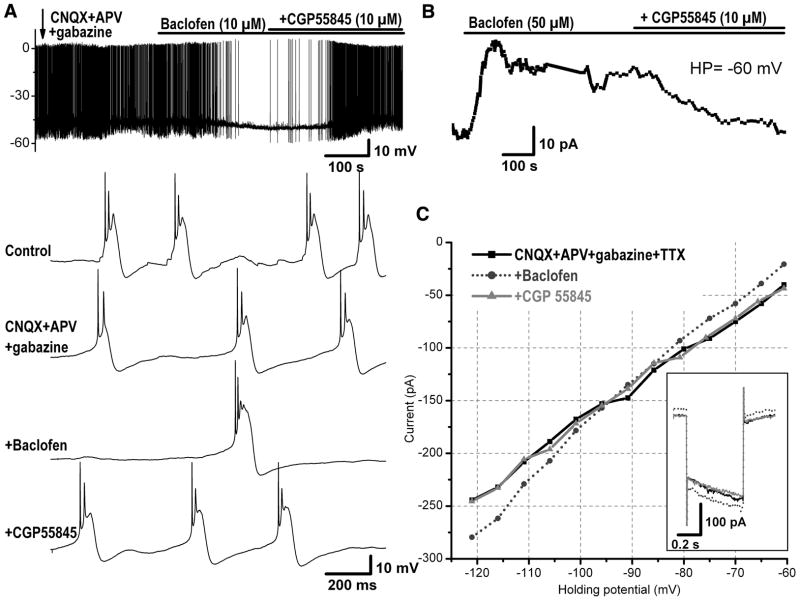
The previous results indicate that activation of GABAB-Rs on ET cells reduces the overall activity of these cells by decreasing their firing and interburst frequencies; however, it paradoxically strengthens the bursts by increasing the number of spikes/burst and the intraburst frequency. These paradoxical effects of baclofen on the bursting pattern prompted further investigation of the intracellular mechanisms involved, so we recorded from ET cells in whole cell mode and examined the effect of baclofen on the membrane potential or current. In current-clamp mode under normal conditions, baclofen (10 μM) hyperpolarized the membrane potential in three of four ET cells tested by −3.2 ± 0.07 mV, from −50 to −54 mV (n = 3). It is important to note here that the duration of the underlying depolarizing envelope of spike bursts recorded intracellularly (~90 ms, Fig. 3A; see also Hayar et al. 2004b) was much longer than the average duration of spike bursts recorded extracellularly (~40 ms, calculated as the interval between the first and the last spikes in a burst). Baclofen prolonged the burst envelope duration of ET cells by increasing the half-width duration from 50 to 70 ms (n = 3).
FIG. 3.
Direct inhibitory postsynaptic effects of baclofen on ET cells. A: current-clamp recording from an ET cell showing the inhibitory effect of baclofen in the presence of the blockers of fast synaptic transmission (CNQX, 10 μM; APV, 50 μM; and gabazine, 10 μM). Bottom 4 traces: bursting activity at extended timescale under different pharmacological conditions as indicated in the first trace. The fast synaptic blockers eliminated the synaptic potentials that were mostly obvious between bursts in control condition. Baclofen slightly hyperpolarized the membrane potential and significantly decreased and eliminated the bursting frequency. This effect was reversed by addition of the GABAB-R blocker CGP55845. B: another ET cell was recorded in voltage clamp at holding potential (HP) = −60 mV in the presence of tetrodotoxin (TTX, 1 μM) and blockers of fast synaptic transmission (CNQX, 10 μM; APV, 50 μM; gabazine, 10 μM). Baclofen induced an outward current that was reversed by the GABAB-R receptor antagonist CGP55845. C: plot of the current–voltage relationship in the same ET cell as in B under different pharmacological conditions. Baclofen produced an outward current at membrane potentials above −90 mV and an inward current at membrane potentials below −90 mV, which is near the reversal potential of potassium channels. Inset: current response to a 50-mV hyperpolarizing voltage step from −60 to −110 mV (duration 0.5 s) under different pharmacological conditions. During baclofen application, a larger current (dotted trace) was produced in response to the same voltage step, indicating an increase in conductance (i.e., decrease in resistance) of the cell in response to GABAB-R activation.
The previous results do not rule out the possibility that baclofen may indirectly exert its effects, at least in part, by inhibiting action potential–dependent release of neurotransmitters that act slowly via metabotropic receptors (e.g., glutamatergic, serotonergic, and dopaminergic receptors). To investigate whether baclofen exerts direct effects on ET cells, we further blocked action potentials by application of the sodium channel blocker TTX, in addition to blockade of fast glutamatergic and GABAergic transmission. Baclofen was tested in voltage-clamp mode at HP of −60 mV during application of CNQX + APV + gabazine + TTX. At 10 μM, baclofen produced no significant effect on the holding current, whereas at 50 μM, it induced an outward current of 16 ± 3.3 pA (n = 7, P < 0.01, Fig. 3B). Such outward current corresponded to a hyperpolarization of the resting membrane potential by 8.0 ± 1.9 mV (from −55.1 ± 3.5 to −63.1 ± 2.9 mV, calculated as the voltage level at which no current was injected in the cell, n = 7). We also tested baclofen on the membrane input resistance, which was calculated as the slope of linear regression fit of the current–voltage relationship. In the presence of CNQX + APV + gabazine + TTX (control), baclofen significantly decreased the input resistance, an effect that was partially reversed by additional application of CGP55845 (control, 625 ± 97 MΩ; baclofen, 536 ± 87 MΩ; CGP55845, 611 ± 100 MΩ; n = 7; P < 0.05, Fig. 3C). The current activated by baclofen tended to reverse at holding potentials ranging from −85 to −95 mV, close to the equilibrium potential of potassium ions, suggesting the involvement of potassium channels in the GABAB-R–mediated response. Taken together these results suggest the presence on ET cells of functional postsynaptic GABAB-Rs that can be directly activated by baclofen. Although baclofen produced relatively small changes on the membrane potential and current of ET cells, these changes seem to lead to prominent modulation of the bursting pattern of ET cells. The relatively significant effects of baclofen (10 μM) on the bursting compared with its insignificant effect on the membrane current could be due to additional GABAB-R–mediated modulation of voltage-dependent channels that are not active at a holding potential of −60 mV. It is also possible that baclofen responses were reduced in whole cell recordings due to a decrease in the affinity of GABAB-Rs because of washout of intracellular messengers involved in the transduction mechanism of these receptors.
Baclofen inhibits spontaneous and evoked EPSCs in JG cells
The effects of baclofen on the mode of bursting and on the membrane potential of ET cells may lead to a modulation of dendrodendritic synaptic transmission between ET cells and other JG neurons. Most of the spontaneous EPSCs in PG and SA cells occur in bursts and our previous results (Hayar et al. 2004a) suggest that they originate at least in part from action potential–dependent propagation in ET cell dendrites. To test whether GABAB-Rs modulate ET-to-(PG or SA) cell dendrodendritic transmission, we first recorded spontaneous bursts of EPSCs in PG or SA cells (which responded disynaptically to olfactory nerve stimulation by a relatively long and variable latency; Hayar et al. 2004a) in voltage-clamp mode at HP = −60 mV without addition of any blocker (Fig. 4). Baclofen blocked almost completely the spontaneous bursts of EPSCs and reduced the frequency of all EPSCs in PG and SA cells from 11.7 ± 4.1 to 5.2 ± 3.2 Hz (n = 6, P = 0.05). The average spontaneous EPSC amplitude was also reduced by baclofen from 34 ± 4 to 27 ± 3 pA (n = 6, P < 0.05). These data suggest that baclofen reduced dendrodendritic spontaneous glutamatergic release onto JG interneurons.
FIG. 4.
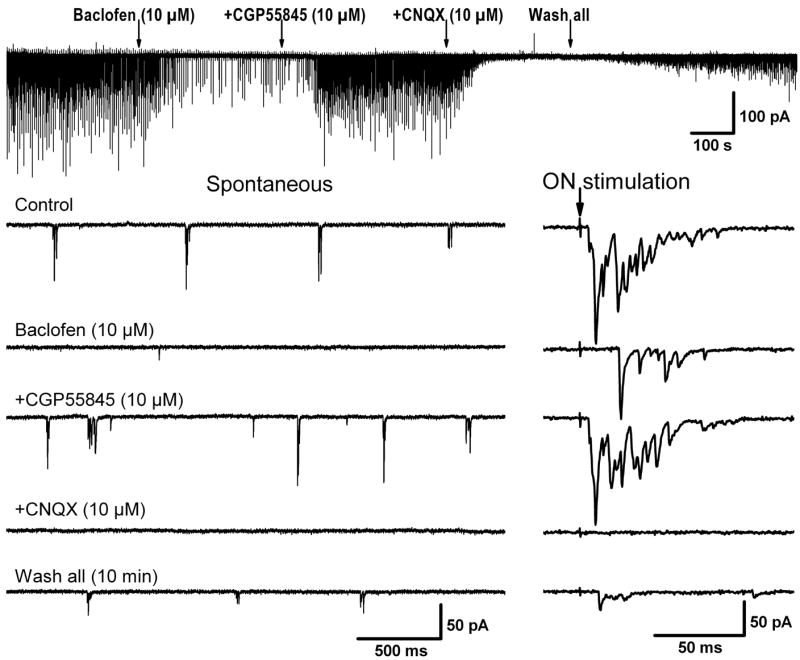
Baclofen effects on spontaneous and evoked bursts of excitatory postsynaptic currents (EPSCs) in periglomerular (PG) cells. All traces were obtained from the same PG cell recorded in voltage clamp at HP = −60 mV. Baclofen blocked almost completely the spontaneous bursts of EPSCs and the olfactory nerve–evoked bursts of EPSCs were either eliminated (failure, not shown) or reduced in amplitude. Baclofen also increased the latency of the EPSC bursts. These effects were reversed after addition of the GABAB-R blocker CGP55845 to the bath. The spontaneous and evoked EPSCs were blocked by the non–N-methyl-D-aspartate receptor blocker CNQX. A partial recovery of the EPSCs was obtained after washout of the drugs.
We next stimulated the olfactory nerve layer to evoke disynaptic bursts of EPSCs in PG or SA cells. The evoked bursts of EPSCs resulted from direct activation of ET cells by olfactory nerve axons and subsequent release of glutamate from ET cells via direct synaptic coupling between ET and PG cells (Hayar et al. 2004a). The olfactory nerve–evoked bursts of EPSCs were either eliminated (i.e., failure) by baclofen or reduced in peak amplitude (calculated after averaging the compound evoked EPSCs in 10 trials in each condition), which on average decreased from 125 ± 26 to 26 ± 6.4 pA (n = 6, P < 0.01). Baclofen increased the average latency from 6.7 ±2.1 to 10.3 ± 1.3 ms (n = 6, P < 0.05) and the average time-to-peak of the evoked EPSC bursts from 22.7 ± 8.4 to 70.1 ± 17.8 ms (n = 6, P < 0.01; calculated between the stimulating pulse and the maximal amplitude of the average evoked response) (Fig. 4). These results suggest that the olfactory nerve–evoked bursts of EPSCs were not monosynaptically driven and that the increase in delay was probably caused by the longer time needed for the monosynaptically evoked excitatory postsynaptic potentials (EPSPs) in ET cells to reach threshold for triggering spike bursts (Hayar et al. 2004a). The effects of baclofen were reversed after addition of the GABAB-R blocker CGP55845 to the bath. Taken together, these results indicate that activation of GABAB-Rs has a considerable inhibitory effect on the polysynaptic pathway involving glutamatergic transmission from olfactory nerve axons to ET cells and then to PG cells. However, at this stage, it is unknown whether these effects are due to direct inhibition of ET cell bursting by baclofen (previous results) or due to direct inhibition of glutamatergic release from ET cell dendrites. To resolve this issue, we investigated the effects of baclofen on action potential–independent EPSCs in PG or SA cells.
The spontaneous EPSCs recorded in PG and SA cells correspond to the sum of two types of synaptic activity. One is resistant to TTX and corresponds to the action potential–independent spontaneous release of glutamate at synapses (mEPSCs). The other type of activity is TTX sensitive because it is evoked by action potential propagation in dendrites of spontaneously bursting ET cells that are presynaptic to the recorded PG and SA cells (Hayar et al. 2004a). Because of the widespread dendrodendritic interactions within the glomeruli (Pinching and Powell 1971a,b), action potential–dependent EPSCs originate mostly from the tufted dendrites of ET and mitral/tufted cells that are associated with the same glomerulus. TTX considerably reduced (88 ± 3% reduction) the frequency of spontaneous EPSCs in PG and SA cells from 20 ± 5 to 1.7 ± 0.6 Hz (n = 9, P < 0.01; not shown). This result indicates that almost all spontaneous EPSCs in these cells are action potential dependent, unlike the EPSCs of ET cells that are mostly (~80%) TTX resistant (Hayar et al. 2004b).
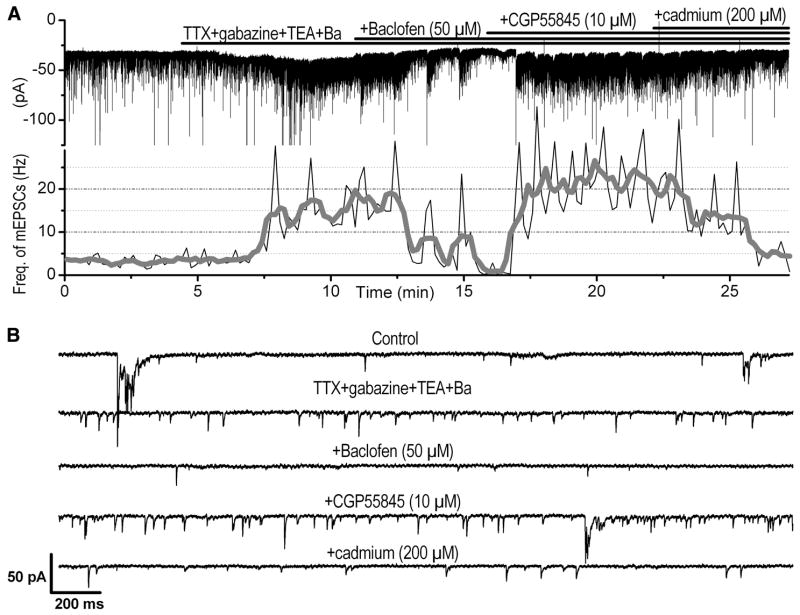
The relatively low frequency of mEPSCs in PG and SA cells precluded the possibility of further examining an additional inhibitory effect of baclofen. Additionally, we observed that application of 50 μM baclofen induced an outward current of ≤ 20 pA in some PG cells during TTX application (not shown). Therefore it is possible that under these conditions baclofen could activate potassium channels leading to a decrease in resistance, which might affect the detectability of mEPSCs. To circumvent these potential problems, we filled the patch pipette with a solution that contains the potassium and sodium channel blockers, cesium and QX-314, respectively. Moreover, we enhanced spontaneous glutamatergic release that is independent of spiking activity by applying, in addition to TTX and gabazine, the potassium channel blockers TEA (10 mM) and barium (1 mM). The frequency of mEPSCs in this cocktail of blockers was considerably higher (11.3 ± 4.7 Hz, n = 6) than that during application of TTX alone (P < 0.01, unpaired t-test). It should also be noted that mEPSC activity in the presence of this cocktail of blockers became highly irregular with frequent changes in mEPSC frequency and amplitude. Nevertheless, under these conditions, baclofen (50 μM) produced significant inhibitory effects on mEPSCs (Fig. 5) by reducing their frequency from 11.3 ± 4.7 to 5.2 ± 2.0 Hz (n = 6, 4 PG cells and 2 SA cells, P < 0.05) without significantly changing the mEPSC amplitude (control, 12.3 ± 1.2 pA; baclofen, 10.9 ± 0.8 pA, n = 6, P = 0.173). The effect of baclofen on the mEPSC frequency was reversed after addition of the GABAB-R blocker CGP55845 (20.6 ± 4.7 Hz, n = 5, P < 0.05). Despite the apparent increase of mEPSC frequency in CGP55845 compared with control before baclofen application, this increase did not reach statistical significance (P = 0.06). Additional application of cadmium (200 μM) significantly decreased the frequency of mEPSCs by 82%, from 20.6 ± 4.7 to 3.6 ± 0.54 Hz (n = 5, P < 0.05). Although not all mEPSCs were suppressed by cadmium, these results suggest that the majority of the mEPSCs inhibited by baclofen were dependent on calcium influx into ET cell dendrites, although we do not exclude the possibility that baclofen might also reduce calcium-independent mEPSCs.
FIG. 5.
Baclofen reduces the frequency of action potential–independent EPSCs [miniature (m)EPSCs]. A, top: current trace of a short axon (SA) cell recorded in voltage clamp at HP = −70 mV with inward deflections, indicating the occurrence of spontaneous EPSCs. The pipette intracellular solution contains cesium and QX-314 (see methods for details). Bottom: corresponding histogram of the frequency of EPSCs throughout the experiment. The histogram was first constructed using a bin width of 10 s (thin line), and then it was further smoothed using adjacent averaging of 5 data points. The histogram was normalized to the bin width to reflect the mean frequency of EPSCs. Note that the increase or decrease in EPSCs was associated with a slight inward or outward current, respectively, suggesting that background EPSC frequency level might contribute to the mean current level. B: sample current traces shown at extended timescale (3 s each) under different pharmacological conditions corresponding to the recording shown in A. Application of the blockers (TTX, 1 μM; gabazine, 10 μM; TEA, 10 mM; barium chloride, 1 mM) significantly increased the EPSC frequency, which became highly irregular. The TTX-resistant mEPSCs were inhibited by baclofen and this effect was reversed by additional application of the GABAB-R blocker CGP55845. Further addition of the calcium channel blocker cadmium blocked most of the mEPSCs.
DISCUSSION
This study demonstrates the presence of functional postsynaptic GABAB-Rs on ET cells. Activation of these receptors modulates the bursting pattern of ET cells and significantly decreases the release of glutamate from their dendrites onto PG and SA cells.
GABAB-Rs are heterodimers consisting of two distinct subunits (for review, see Bettler et al. 2004; Emson 2007). The GABAB1 subunit contains the ligand-binding pocket, whereas the GABAB2 subunit is responsible for translocation of the heterodimer from the endoplasmic reticulum to the membrane. Two variants of the GABAB1 subunit have been cloned: GABAB1a and GABAB1b. A pre- versus postsynaptic localization for each isoform could not be directly demonstrated because of the lack of selective ligands. Using knockout mice for the two isoforms, Pérez-Garci et al. (2006) showed that postsynaptic inhibition of dendritic calcium spikes of layer 5 pyramidal neurons of the somatosensory cortex is mediated by GABAB1b receptors, whereas presynaptic inhibition of GABA release is mediated by GABAB1a receptors. It is unknown whether the postsynaptic GABAB-Rs on ET cells differ from the presynaptic receptors located on the olfactory nerve terminals. Nevertheless, previous studies have provided evidence for the existence of postsynaptic GABAB-Rs in the glomerular layer, although the cell types expressing such receptors have not been clearly identified. For example, immunocytochemical studies (Bonino et al. 1999) have shown the presence of postsynaptic GABAB-Rs on JG neurons using an antibody for both splice variants of the receptors (GABAB1a and GABAB1b). Additional evidence was obtained recently using ultrastructural electron microscopy of the subcellular localization of the GABAB-Rs. Using a subunit-specific antibody of the GABAB2 subunit and preembedding immunogold labeling, it was shown that postsynaptic, and perhaps presynaptic, GABAB-Rs may be expressed at GABAergic synapses between dendrites of PG interneurons and projection neurons (Kratskin et al. 2006).
The interactions of excitatory and inhibitory inputs on ET cell dendrites provide a rich source of computation possibilities. In a previous study, we showed that ET cell bursting is controlled and coordinated by fast excitatory and inhibitory synaptic inputs, which strengthen and weaken bursting, respectively, by modulating the number of spikes/burst (Hayar and Ennis 2007). Therefore ET cell excitatory and inhibitory inputs may be encoded as a change in the pattern of spike bursting. In this study, we showed that activation of GABAB-Rs decreased the bursting frequency but it increased the burst duration and the number of spikes/burst. These effects were obtained in the presence of fast synaptic blockers and are therefore most likely due to postsynaptic modulation of membrane channels. The decrease in bursting frequency could be explained in part by hyperpolarization of the membrane potential, which was observed in whole cell recordings. In contrast to our results, baclofen has been reported not to alter the input resistance and the membrane current or potential of periglomerular cells (Aroniadou-Anderjaska et al. 2000) and of mitral and granule cells (Isaacson and Vitten 2003). In this study, we included BAPTA in the pipette because the apparent affinity of GABAB-Rs may be reduced if BAPTA is not used to maintain internal calcium <70 nM (Shen and Slaughter 1999). It is therefore possible that a postsynaptic effect of baclofen on other types of olfactory bulb neurons might not have been detected in previous studies because of the inadequate buffering of intracellular calcium. The lack of effect of baclofen could also be due to intracellular dialysis of some intracellular messengers involved in the transduction mechanism of GABAB-Rs.
GABAB-Rs have been implicated in the modulation of inwardly rectifying, barium-sensitive potassium channels (Gahwiler and Brown 1985; Inoue et al. 1985; Ogata 1990; Rossi et al. 2006). Our results indicate that baclofen induced an outward current and hyperpolarized the membrane potential of ET cells in the presence of blockers of fast synaptic transmission. These effects may therefore be due to direct activation of postsynaptic GABAB-Rs leading to the opening of inwardly rectifying potassium channels. The membrane hyperpolarization by baclofen may be responsible in part for the increase in the number of spikes/burst. We have shown in a previous study (Hayar et al. 2004b) that intracellular injection of negative current could reproduce the same observation (i.e., decrease in interburst frequency and increase in the number of spikes/ burst).
GABAB-Rs are also involved in the modulation of calcium channels (Bussières and Manira 1999; Heidelberger and Matthews 1991; Maguire et al. 1989; Tatebayashi and Ogata 1992). An increase in ET cell burst duration by activation of GABAB-Rs may result from inhibition of calcium channels. In the olfactory bulb, GABAB-R–mediated inhibition of calcium channels was shown to occur in olfactory nerve terminals (Wachowiak et al. 2005) and granule cells (Isaacson and Vitten 2003). We previously showed that the calcium channel blockers cadmium and nickel increased burst duration in ET cells (Hayar et al. 2004b); similar results were also obtained in pyramidal CA1 neurons (Azouz et al. 1996). It is possible that the burst is normally terminated by a calcium-dependent potassium conductance. In this case, an inhibition of calcium influx would inhibit the calcium-activated slow afterhyperpolarization that contributes to burst termination. This interpretation is supported by our finding that baclofen reduced spike firing adaptation in the burst, which was quantified by the ISI slope.
Previous results showed that unlike ET cells, SA cells and most PG cells lack monosynaptic olfactory nerve input (Hayar et al. 2004a; Tyler et al. 2007). Simultaneous recordings from ET and PG cells indicate that ET cells provide monosynaptic, excitatory, glutamatergic input to SA and PG cells of the same glomerulus (Hayar et al. 2004a). This excitatory drive is most likely mediated by dendrodendritic synapses because the morphological data show that ET cell axons exit the glomerular layer with no discernible intraglomerular branching (Hayar et al. 2004a,b). Therefore ET cells are the predominant relay of olfactory nerve input to PG and SA cells and most EPSCs in PG and SA cells derive from ET cell dendritic glutamate release. Contradictory results exist in the literature with respect to the effect of baclofen on olfactory nerve–evoked EPSCs in PG cells. Some studies suggested that GABAB-Rs may be present on all olfactory nerve terminals (Aroniadou-Anderjaska et al. 2001; Murphy et al. 2005). However, one study (Belluzzi et al. 2004) found no evidence for the presence of functional GABAB-Rs on olfactory terminals that contact PG cells. These controversial results could be due to the lack of distinction between neurons that receive direct or indirect input from olfactory nerve (Hayar et al. 2004a) and because PG might be sometimes confused with ET cells as a result of the size overlap between the two groups of cells.
In this study, we tested baclofen specifically on PG cells that have no direct connection to the olfactory nerve by selecting those cells that responded to olfactory nerve with a relatively long and variable latency burst of EPSCs (Hayar et al. 2004a). We found that baclofen almost completely inhibited olfactory nerve–evoked EPSC bursts in PG cells. These evoked EPSC bursts are produced disynaptically via initial activation of ET cells, which generate a burst of spikes and subsequently release glutamate from their dendrites onto PG cells. In this case, the observed strong inhibition of baclofen on the evoked bursts of EPSCs could occur at many levels: 1) presynaptic inhibition of olfactory axons that directly innervate the ET cells, 2) postsynaptic inhibition by activation of GABAB-Rs on ET cells, and 3) inhibition of glutamate release from ET cell to PG cell dendrites.
To specifically investigate the last mechanism, we tested baclofen on mEPSCs in the presence of TTX to block action potential–dependent synaptic release and the potassium channel blockers TEA and barium to block inwardly rectifying potassium channels that might be activated by GABAB-Rs. In the presence of these sodium and potassium channel blockers, baclofen significantly reduced the mEPSCs in PG and SA cells. Because most of these mEPSCs (82%) were blocked by cadmium, these results provide indirect evidence that activation of GABAB-Rs may reduce calcium influx into the ET cell dendrites, which then release less glutamate onto PG and SA cells.
It is possible that activation of potassium channels by GABAB-Rs could be a sufficient mechanism to explain the inhibition of transmitter release from ET to PG and SA cells. However, our experiments on mEPSCs were performed in the presence of the potassium channel blockers: cesium applied intracellularly in the pipette solution and TEA and barium applied extracellularly in the bath solution. Since sodium channels were also blocked by TTX in these experiments, we assume that under these conditions, voltage-dependent calcium channels were the only active channels. Therefore by exclusion, our data suggest that the decrease of mEPSCs by baclofen may involve calcium channels, assuming that the potassium channel blockers—in particular, barium—has blocked the inwardly rectifying potassium channels that were activated by GABAB-Rs (Gahwiler and Brown 1985; Inoue et al. 1985; Ogata 1990; Rossi et al. 2006). Despite the overall inhibitory effect of baclofen on ET cell firing activity, its paradoxical increase of the number of spikes/bursts would lead to facilitation of transmitter release since additional spikes are expected to increase the probability of synaptic release due to an increase in calcium influx in the dendrites (Murphy et al. 2005; Zhou et al. 2006). This indicates that an additional mechanism other than opening of potassium channels may be involved in the reduction of transmitter release by baclofen. We propose that the GABAB-R–mediated inhibition of dendrodendritic transmitter release may result not only from the effects of activation of potassium channels but also from the inhibition of calcium channels. However, direct evidence for inhibition of ET cell calcium currents by baclofen (unpublished data; Karpuk and Hayar 2007) would be required to fully support this hypothesis.
Our findings are in contrast with the absence of effect of baclofen on glutamate release from the lateral dendrites of mitral and tufted cells. In one study, the field potential recorded in the granule cell layer in response to lateral olfactory tract stimulation was not affected by baclofen (Aroniadou-Anderjaska et al. 2000). In another study, baclofen exerted only a modest inhibitory role on mitral cell dendritic glutamate release but it strongly reduced GABA release evoked from granule cells by inhibiting their high-voltage–activated calcium channels (Isaacson and Vitten 2003). Therefore the relatively strong inhibition of the ET-to-PG cell dendrodendritic glutamate release, as found in this study, might have important functional significance in the glomeruli that serve as the initial site for processing sensory input. We have recently shown that glomerular field potentials are generated mainly by JG neurons with little contribution from mitral/tufted cells. This is because the glomerular field potentials had comparable properties in standard horizontal slices, and in isolated glomerular layer slices, where only JG cells were preserved (Karnup et al. 2006). It is therefore possible that the strong inhibition by baclofen of evoked glomerular field potential (Aroniadou-Anderjaska et al. 2000) could be due, in large part, to inhibition of recurrent glutamate release from ET cell dendrites.
Important questions that arise from this study are when and how the postsynaptic GABAB-Rs on ET cells are activated? Despite the high potency of CGP55845 to reverse the effects of baclofen, we did not observe any significant postsynaptic effect of this antagonist when applied alone. GABAB-Rs could be activated by taurine, which is colocalized with glutamate in olfactory nerve axons (Didier et al. 1994). However, the postsynaptic effects of taurine on ET cells were blocked almost completely by GABAA receptor antagonists (Belluzzi et al. 2004). Because GABAB-Rs are mostly extrasynaptic (Kratskin et al. 2006), they might primarily be activated during bursts of synaptic input, when a high concentration of GABA is released in the synaptic cleft. This is likely to occur under physiological conditions because ET cells exhibit spontaneous rhythmic bursts of IPSCs (Hayar et al. 2005). These bursts of IPSCs may reflect the temporal and spatial summation of inhibitory inputs originating from several presynaptic PG cells. Because inhibitory input often occurs after an initial excitatory input (Hayar et al. 2005), activation of ET cell GABAA- and GABAB-Rs may limit the amount of glutamate released by ET cells and, as a consequence, may reduce the amount of glutamate spillover in the glomerulus.
In the vertebrate olfactory bulb, GABAB-R activation in the olfactory bulb seems to make output cell responses more salient by reducing their background activity while maintaining most of their odor response (Duchamp-Viret et al. 2000; Palouzier-Paulignan et al. 2002). The GABAB-R–mediated tonic inhibition of glutamate release from olfactory nerve terminals (Aroniadou-Anderjaska et al. 2000) may sharpen the spatial pattern of odor-activated glomeruli and facilitate detection of the predominant odor. Blockade of olfactory bulb GABAB-Rs induced aversive olfactory behavior in young rats during the presentation of new odors, suggesting a role of these receptors in olfactory learning (Okutani et al. 2003). Blocking presynaptic inhibition in vivo increased the amplitude of odorant-evoked input to glomeruli but had little effect on spatial patterns of glomerular input (McGann et al. 2005). The presynaptic inhibition by GABAB-Rs, whether at the level of olfactory nerve terminals or at the level of ET-to-PG cell dendrodendritic synapse, may serve as a means of fine-tuning synaptic strength or as negative feedback to prevent excessive transmitter release to influence the firing of postsynaptic neurons. Therefore the presence of a functional GABAB-R–mediated presynaptic inhibition, as shown by several previous studies, and the GABAB-R–mediated postsynaptic inhibition, as shown by this study, may regulate the strength of olfactory input to the CNS while maintaining spatial representations of odor information.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health Grants DC-06356, DC-07123, and RR-020146.
References
- Antal M, Eyre M, Finklea B, Nusser Z. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur J Neurosci. 2006;24:1124–1136. doi: 10.1111/j.1460-9568.2006.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Ault B, Gruenthal M, Armstrong DR, Nadler JV, Wang CM. Baclofen suppresses bursting activity induced in hippocampal slices by differing convulsant treatments. Eur J Pharmacol. 1986;126:289–292. doi: 10.1016/0014-2999(86)90059-2. [DOI] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492:211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O, Puopolo M, Benedusi M, Kratskin I. Selective neuroinhibitory effects of taurine in slices of rat main olfactory bulb. Neuroscience. 2004;124:929–944. doi: 10.1016/j.neuroscience.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bonino M, Cantino D, Sassoe-Pognetto M. Cellular and subcellular localization of gamma-aminobutyric acid B receptors in the rat olfactory bulb. Neurosci Lett. 1999;274:195–198. doi: 10.1016/s0304-3940(99)00697-7. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Bussières N, El Manira A. GABA(B) receptor activation inhibits N- and P/Q-type calcium channels in cultured lamprey sensory neurons. Brain Res. 1999;847:175–185. doi: 10.1016/s0006-8993(99)02002-8. [DOI] [PubMed] [Google Scholar]
- Chen Q, Pan HL. Regulation of synaptic input to hypothalamic presympathetic neurons by GABA(B) receptors. Neuroscience. 2006;142:595–606. doi: 10.1016/j.neuroscience.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34:341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Cui LN, Coderre E, Renaud LP. GABA(B) presynaptically modulates suprachiasmatic input to hypothalamic paraventricular magnocellular neurons. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1210–R1216. doi: 10.1152/ajpregu.2000.278.5.R1210. [DOI] [PubMed] [Google Scholar]
- Didier A, Ottersen OP, Storm-Mathisen J. Differential subcellular distribution of glutamate and taurine in primary olfactory neurones. Neuroreport. 1994;6:145–148. doi: 10.1097/00001756-199412300-00037. [DOI] [PubMed] [Google Scholar]
- Duchamp-Viret P, Delaleu JC, Duchamp A. GABA(B)-mediated action in the frog olfactory bulb makes odor responses more salient. Neuroscience. 2000;97:771–777. doi: 10.1016/s0306-4522(00)00055-5. [DOI] [PubMed] [Google Scholar]
- Emson PC. GABA(B) receptors: structure and function. Prog Brain Res. 2007;160:43–57. doi: 10.1016/S0079-6123(06)60004-6. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Synaptic actions on mitral and tufted cells elicited by olfactory nerve volleys in the rabbit. J Physiol. 1975;251:497–522. doi: 10.1113/jphysiol.1975.sp011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Ennis M. Endogenous GABA and glutamate finely tune the bursting of olfactory bulb external tufted cells. J Neurophysiol. 2007;98:1052–1056. doi: 10.1152/jn.01214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci. 2004a;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004b;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci. 2005;25:8197–8208. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Inhibition of calcium influx and calcium current by γ-aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci USA. 1991;88:7135–7139. doi: 10.1073/pnas.88.16.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Matsuo T, Ogata N. Possible involvement of K+ -conductance in the action of gamma-aminobutyric acid in the guinea-pig hippocampus. Br J Pharmacol. 1985;86:515–524. doi: 10.1111/j.1476-5381.1985.tb08923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Vitten H. GABA(B) receptors inhibit dendrodendritic transmission in the rat olfactory bulb. J Neurosci. 2003;23:2032–2039. doi: 10.1523/JNEUROSCI.23-06-02032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnup SV, Hayar A, Shipley MT, Kurnikova MG. Spontaneous field potentials in the glomeruli of the olfactory bulb: the leading role of juxtaglomerular cells. Neuroscience. 2006;142:203–221. doi: 10.1016/j.neuroscience.2006.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuk N, Hayar A. Activation of postsynaptic GABAB receptors directly modulates the bursting pattern and synaptic activity of olfactory bulb juxtaglomerular neurons. Soc Neurosci Abstr. 2007:790.12. doi: 10.1152/jn.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuk N, Vorobyov V. Spike sequences and mean firing rate in rat neocortical neurons in vitro. Brain Res. 2003;973:16–30. doi: 10.1016/s0006-8993(03)02478-8. [DOI] [PubMed] [Google Scholar]
- Keller A, Yagodin S, Aroniadou-Anderjaska V, Zimmer LA, Ennis M, Sheppard NF, Jr, Shipley MT. Functional organization of rat olfactory bulb glomeruli revealed by optical imaging. J Neurosci. 1998;18:2602–2612. doi: 10.1523/JNEUROSCI.18-07-02602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratskin I, Kenigfest N, Rio JP, Djediat C, Reperant J. Immunocytochemical localization of the GABAB2 receptor subunit in the glomeruli of the mouse main olfactory bulb. Neurosci Lett. 2006;402:121–125. doi: 10.1016/j.neulet.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin F. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci USA. 1989;86:10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- Nawroth JC, Greer CA, Chen WR, Laughlin SB, Shepherd GM. An energy budget for the olfactory glomerulus. J Neurosci. 2007;27:9790–9800. doi: 10.1523/JNEUROSCI.1415-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell WT, Behbehani MM, Shipley MT. Evidence for GABAB-mediated inhibition of transmission from the olfactory nerve to mitral cells in the rat olfactory bulb. Brain Res Bull. 1994;35:119–123. doi: 10.1016/0361-9230(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Ogata N. Pharmacology and physiology of GABAB receptors. Gen Pharmacol. 1990;21:395–402. doi: 10.1016/0306-3623(90)90687-h. [DOI] [PubMed] [Google Scholar]
- Okutani F, Zhang JJ, Otsuka T, Yagi F, Kaba H. Modulation of olfactory learning in young rats through intrabulbar GABA(B) receptors. Eur J Neurosci. 2003;18:2031–2036. doi: 10.1046/j.1460-9568.2003.02894.x. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Duchamp-Viret P, Hardy AB, Duchamp A. GABA(B) receptor-mediated inhibition of mitral/tufted cell activity in the rat olfactory bulb: a whole-cell patch-clamp study in vitro. Neuroscience. 2002;111:241–250. doi: 10.1016/s0306-4522(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci. 1971a;9:305–345. doi: 10.1242/jcs.9.2.305. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971b;9:347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Rossi P, Mapelli L, Roggeri L, Gall D, de Kerchove d’Exaerde A, Schiffmann SN, Taglietti V, D’Angelo E. Inhibition of constitutive inward rectifier currents in cerebellar granule cells by pharmacological and synaptic activation of GABA receptors. Eur J Neurosci. 2006;24:419–432. doi: 10.1111/j.1460-9568.2006.04914.x. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Internal calcium modulates apparent affinity of metabotropic GABA receptors. J Neurophysiol. 1999;82:3298–3306. doi: 10.1152/jn.1999.82.6.3298. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Sutch CP, Wilson WA. Attenuation of epileptiform bursting by baclofen: reduced potency in elevated potassium. Exp Neurol. 1986;94:726–734. doi: 10.1016/0014-4886(86)90250-5. [DOI] [PubMed] [Google Scholar]
- Tatebayashi H, Ogata N. GABAB-mediated modulation of the voltage-gated Ca2+ channels. Gen Pharmacol. 1992;23:309–316. doi: 10.1016/0306-3623(92)90088-2. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Petzold GC, Pal SK, Murthy VN. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci. 2007;27:9427–9438. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J Neurosci. 1999;19:8808–8817. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Cui LN, Renaud LP. Pre- and postsynaptic GABA(B) receptors modulate rapid neurotransmission from suprachiasmatic nucleus to parvo-cellular hypothalamic paraventricular nucleus neurons. Neuroscience. 2003;118:49–58. doi: 10.1016/s0306-4522(02)00906-5. [DOI] [PubMed] [Google Scholar]
- Wellis DP, Scott JW. Intracellular responses of identified rat olfactory bulb interneurons to electrical and odor stimulation. J Neurophysiol. 1990;64:932–947. doi: 10.1152/jn.1990.64.3.932. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xiong W, Masurkar AV, Chen WR, Shepherd GM. Dendritic calcium plateau potentials modulate input-output properties of juxtaglomerular cells in the rat olfactory bulb. J Neurophysiol. 2006;96:2354–2363. doi: 10.1152/jn.00003.2006. [DOI] [PubMed] [Google Scholar]