Abstract
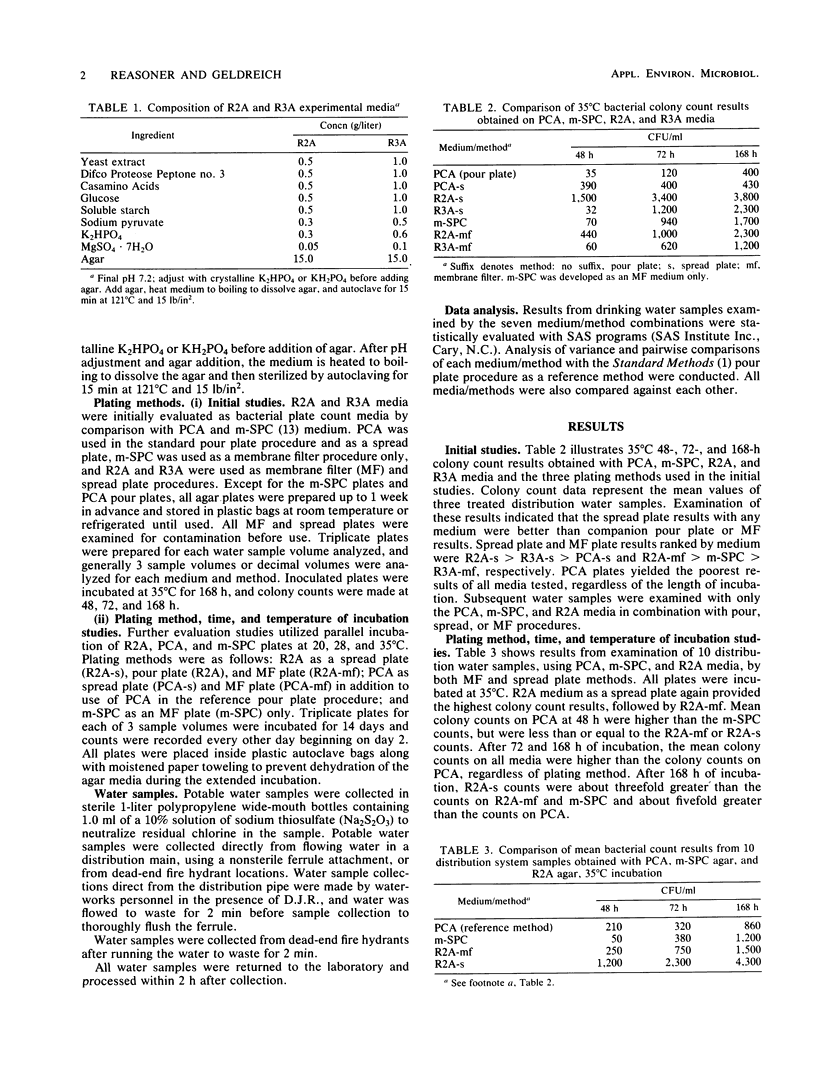
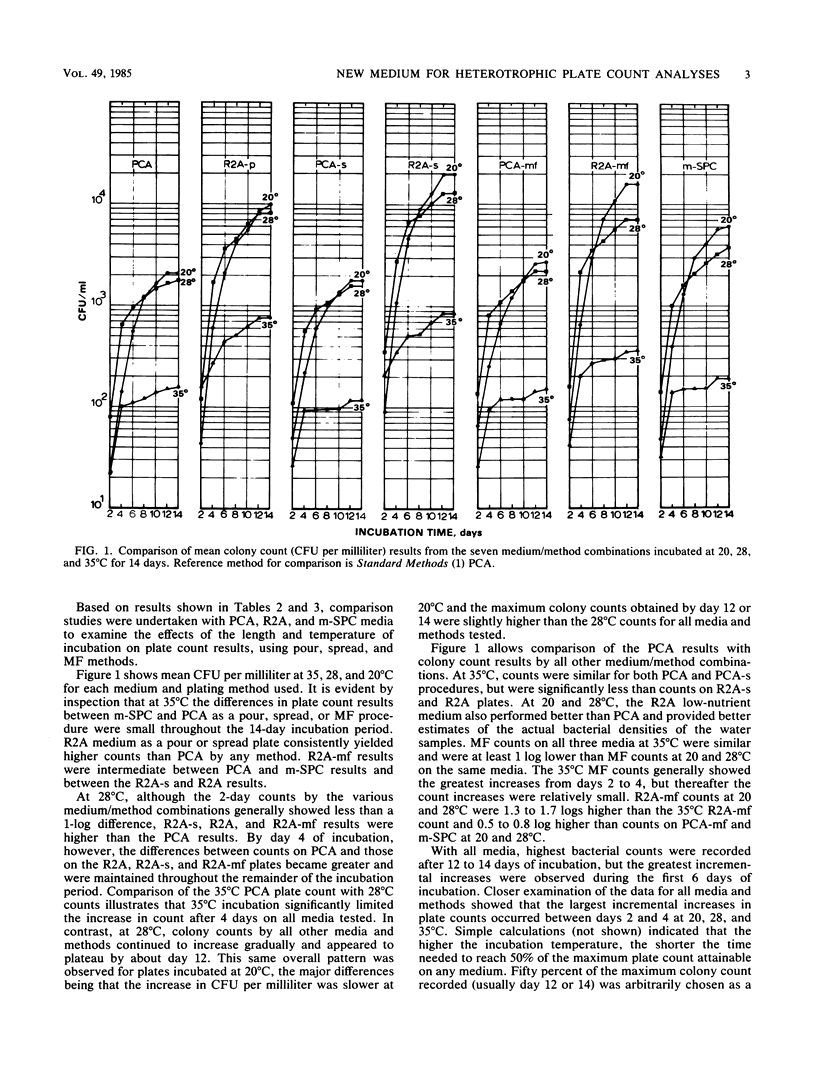
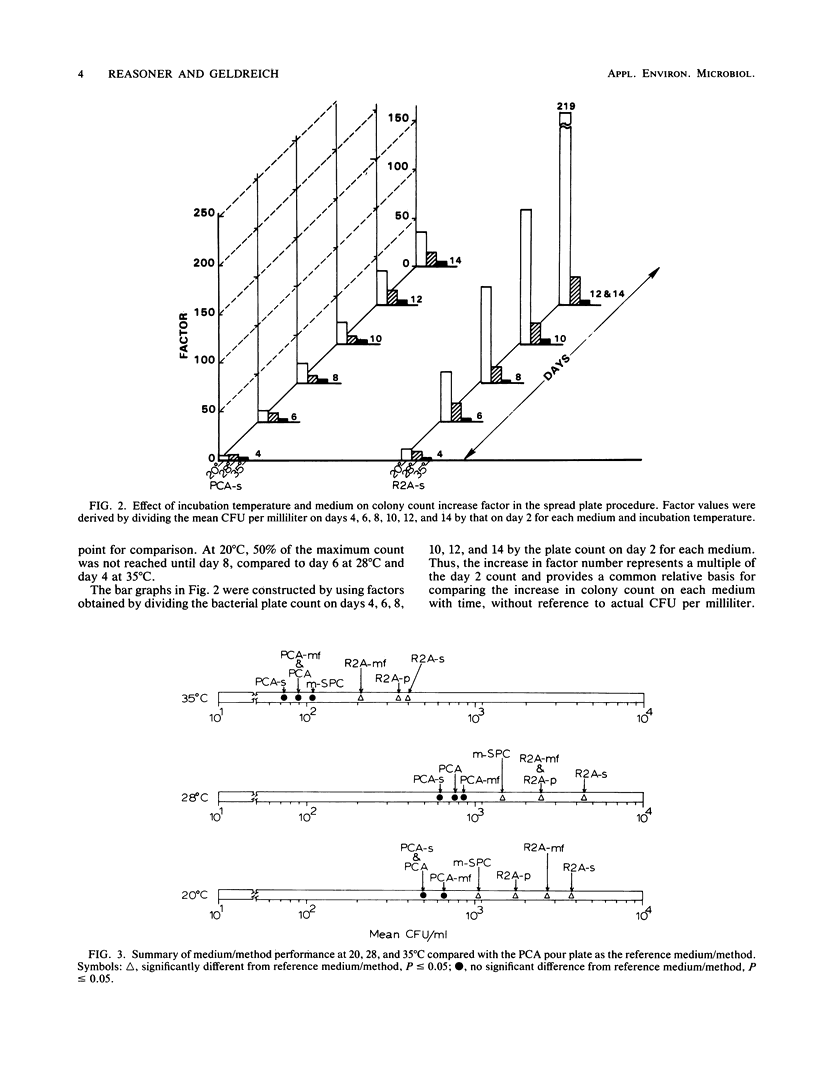
Plate count agar is presently the recommended medium for the standard bacterial plate count (35 degrees C, 48-h incubation) of water and wastewater. However, plate count agar does not permit the growth of many bacteria that may be present in treated potable water supplies. A new medium was developed for use in heterotrophic plate count analyses and for subculture of bacteria isolated from potable water samples. The new medium, designated R2A, contains 0.5 g of yeast extract, 0.5 g of Difco Proteose Peptone no. 3 (Difco Laboratories), 0.5 g of Casamino Acids (Difco), 0.5 g of glucose, 0.5 g of soluble starch, 0.3 g of K2HPO4, 0.05 g of MgSO4 X 7H2O, 0.3 g of sodium pyruvate, and 15 g of agar per liter of laboratory quality water. Adjust the pH to 7.2 with crystalline K2HPO4 or KH2PO4 and sterilize at 121 degrees C for 15 min. Results from parallel studies with spread, membrane filter, and pour plate procedures showed that R2A medium yielded significantly higher bacterial counts than did plate count agar. Studies of the effect of incubation temperature showed that the magnitude of the count was inversely proportional to the incubation temperature. Longer incubation time, up to 14 days, yielded higher counts and increased detection of pigmented bacteria. Maximal bacterial counts were obtained after incubation at 20 degrees C for 14 days. As a tool to monitor heterotrophic bacterial populations in water treatment processes and in treated distribution water, R2A spread or membrane filter plates incubated at 28 degrees C for 5 to 7 days is recommended.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLLINS V. G., WILLOUGHBY L. G. The distribution of bacteria and fungal spores in Blelham Tarn with particular reference to an experimental overturn. Arch Mikrobiol. 1962;43:294–307. doi: 10.1007/BF00405972. [DOI] [PubMed] [Google Scholar]
- Henrici A. T. Studies of Freshwater Bacteria: IV. Seasonal Fluctuations of Lake Bacteria in Relation to Plankton Production. J Bacteriol. 1938 Feb;35(2):129–139. doi: 10.1128/jb.35.2.129-139.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G. Studies on freshwater bacteria: effect of medium composition and method on estimates of bacterial population. J Appl Bacteriol. 1970 Dec;33(4):679–686. doi: 10.1111/j.1365-2672.1970.tb02250.x. [DOI] [PubMed] [Google Scholar]
- Klein D. A., Wu S. Stress: a factor to be considered in heterotrophic microorganism enumeration from aquatic environments. Appl Microbiol. 1974 Feb;27(2):429–431. doi: 10.1128/am.27.2.429-431.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Kelly A., Justice C., Olson B. H. Microbial fouling of reverse-osmosis membranes used in advanced wastewater treatment technology: chemical, bacteriological, and ultrastructural analyses. Appl Environ Microbiol. 1983 Mar;45(3):1066–1084. doi: 10.1128/aem.45.3.1066-1084.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


