Abstract
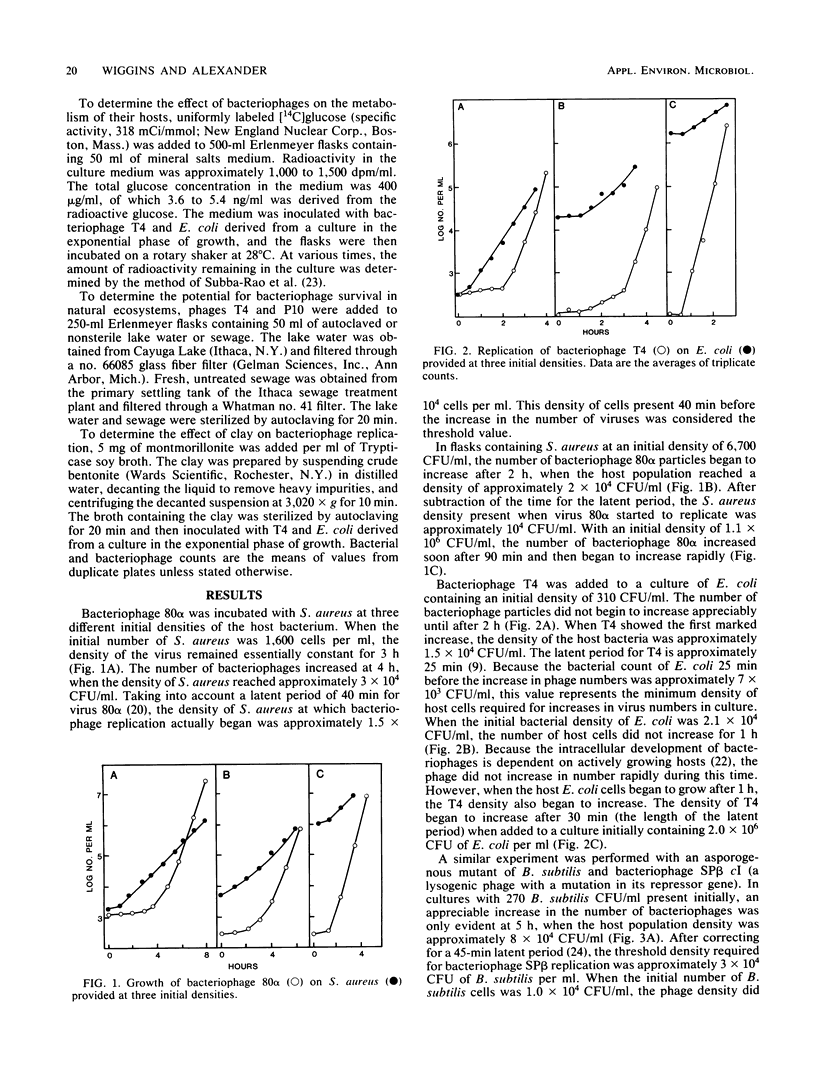
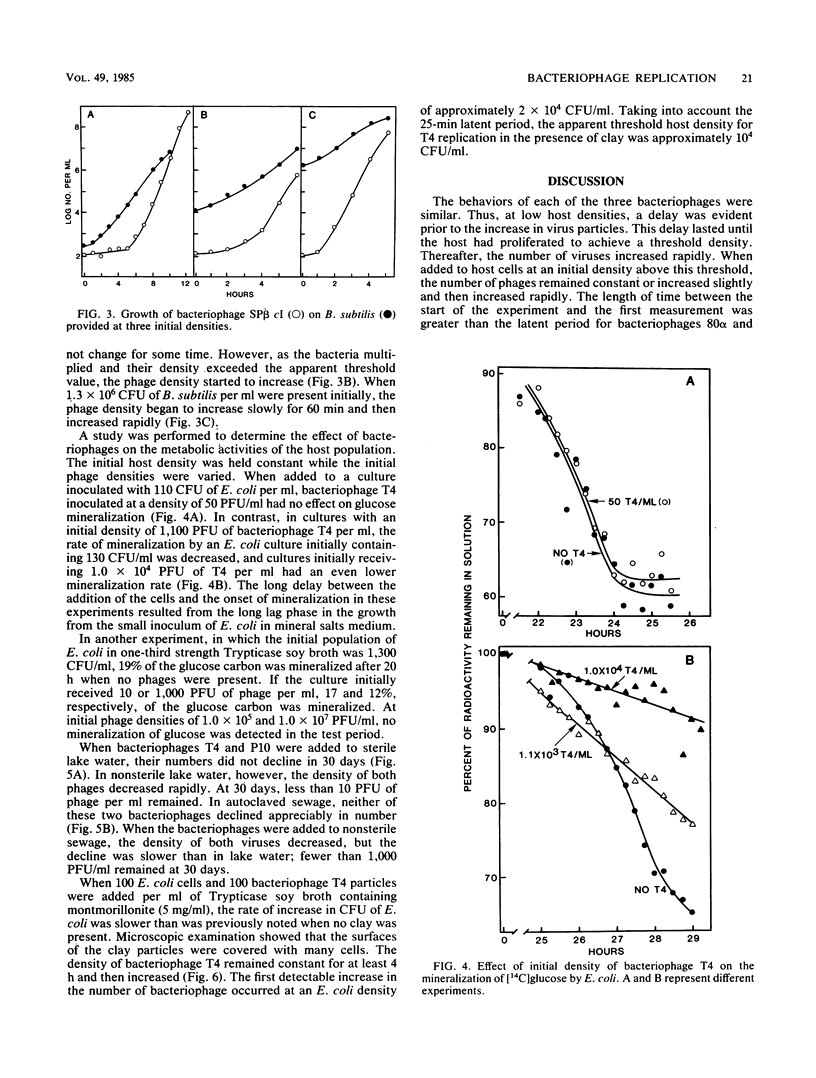
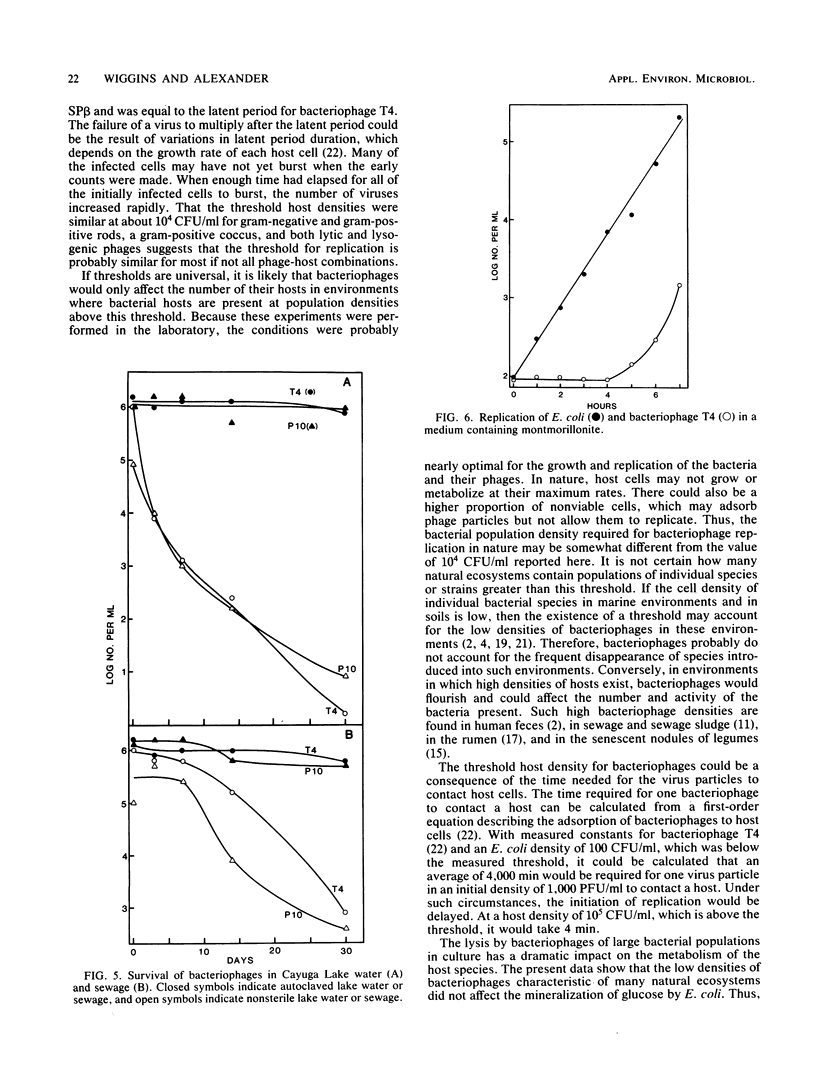
Bacteriophage 80 alpha did not increase in number in cultures containing less than about 1.0 X 10(4) to 1.5 X 10(4) CFU of Staphylococcus aureus per ml, but bacteriophage replication did occur when the number of bacteria exceeded this density, either initially or as a result of host cell multiplication. The minimum density of an asporogenous strain of Bacillus subtilis required for an increase in the number of bacteriophage SP beta cI was about 3 X 10(4) CFU/ml. The threshold density of Escherichia coli for the multiplication of bacteriophage T4 was about 7 X 10(3) CFU/ml. In the presence of montmorillonite, bacteriophage T4 did not increase in number until the E. coli population exceeded 10(4) CFU/ml. The mineralization of glucose was not affected in E. coli cultures inoculated with a low number of bacteriophage T4, but it could not be detected in cultures inoculated with a large number of phage. The numbers of bacteriophage T4 and a bacteriophage that lyses Pseudomonas putida declined rapidly after being added to lake water or sewage. We suggest that bacteriophages do not affect the number or activity of bacteria in environments where the density of the host species is below the host cell threshold of about 10(4) CFU/ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnet Y. M. The effect of rhizobiophages on populations of Rhizobium trifolii in the root zone of clover plants. Can J Microbiol. 1980 May;26(5):572–576. doi: 10.1139/m80-101. [DOI] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. An evaluation of factors affecting the survival of Escherichia coli in sea water. IV. Bacteriophages. Appl Microbiol. 1960 Jul;8:254–256. doi: 10.1128/am.8.4.254-256.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C. W., Clarke N. A. Control of bacteria in nondomestic water supplies. Adv Appl Microbiol. 1966;8:105–143. doi: 10.1016/s0065-2164(08)70494-5. [DOI] [PubMed] [Google Scholar]
- DOERMANN A. H. The intracellular growth of bacteriophages. I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J Gen Physiol. 1952 Mar;35(4):645–656. doi: 10.1085/jgp.35.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso S. K., Keya S. O., Alexander M. Protozoa and the decline of Rhizobium populations added to soil. Can J Microbiol. 1975 Jun;21(6):884–895. doi: 10.1139/m75-131. [DOI] [PubMed] [Google Scholar]
- Evans J., Barnet Y. M., Vincent J. M. Effect of a bacteriophage on the colonisation and nodulation of clover roots by a strain of Rhizobium trifolii. Can J Microbiol. 1979 Sep;25(9):968–973. doi: 10.1139/m79-148. [DOI] [PubMed] [Google Scholar]
- Ewert D. L., Paynter M. J. Technique for determining total bacterial virus counts in complex aqueous systems. Appl Environ Microbiol. 1980 Jan;39(1):253–260. doi: 10.1128/aem.39.1.253-260.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habte M., Alexander M. Protozoa as agents responsible for the decline of Xanthomonas campestris in soil. Appl Microbiol. 1975 Feb;29(2):159–164. doi: 10.1128/am.29.2.159-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- PRETORIUS W. A. Some observations on the role of coliphages in the number of Escherichia coli in oxidation ponds. J Hyg (Lond) 1962 Sep;60:279–281. doi: 10.1017/s0022172400020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Ewert D. L., Chalupa W. Some morphological types of bacteriophages in bovine rumen contents. Appl Microbiol. 1969 Nov;18(5):942–943. doi: 10.1128/am.18.5.942-943.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. INDIGENOUS MARINE BACTERIOPHAGES. J Bacteriol. 1960 Apr;79(4):614–614. doi: 10.1128/jb.79.4.614-614.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


