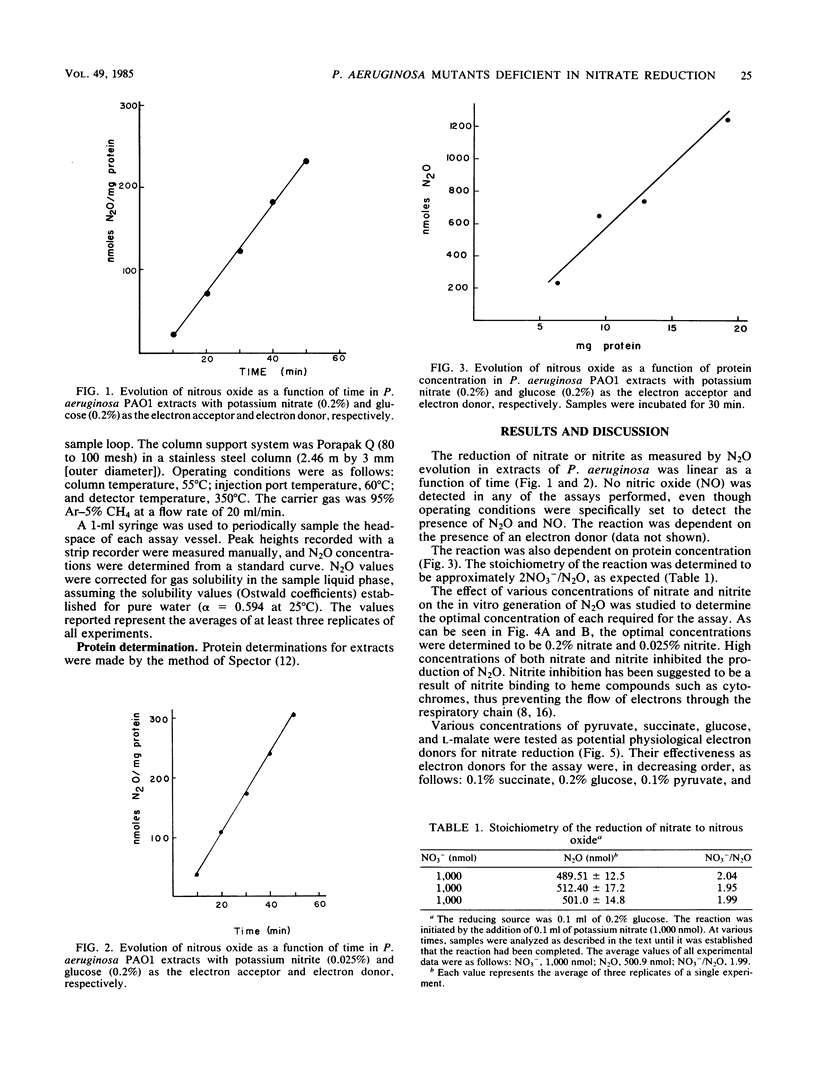
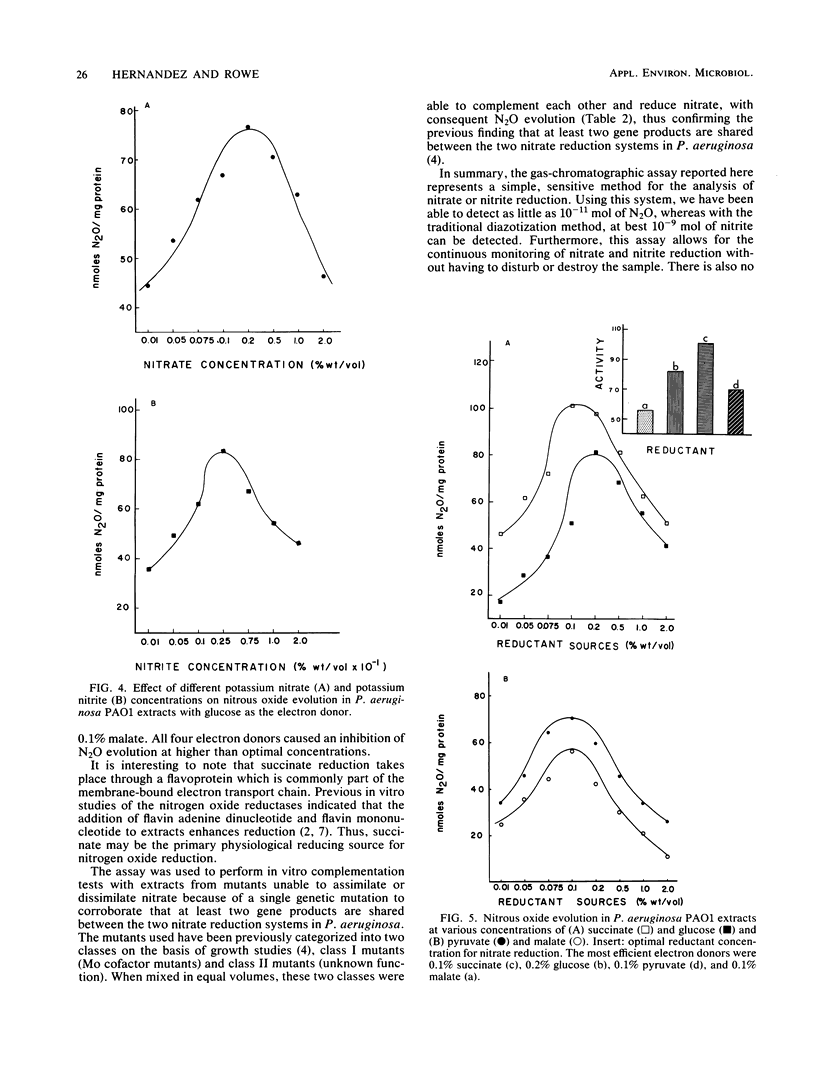
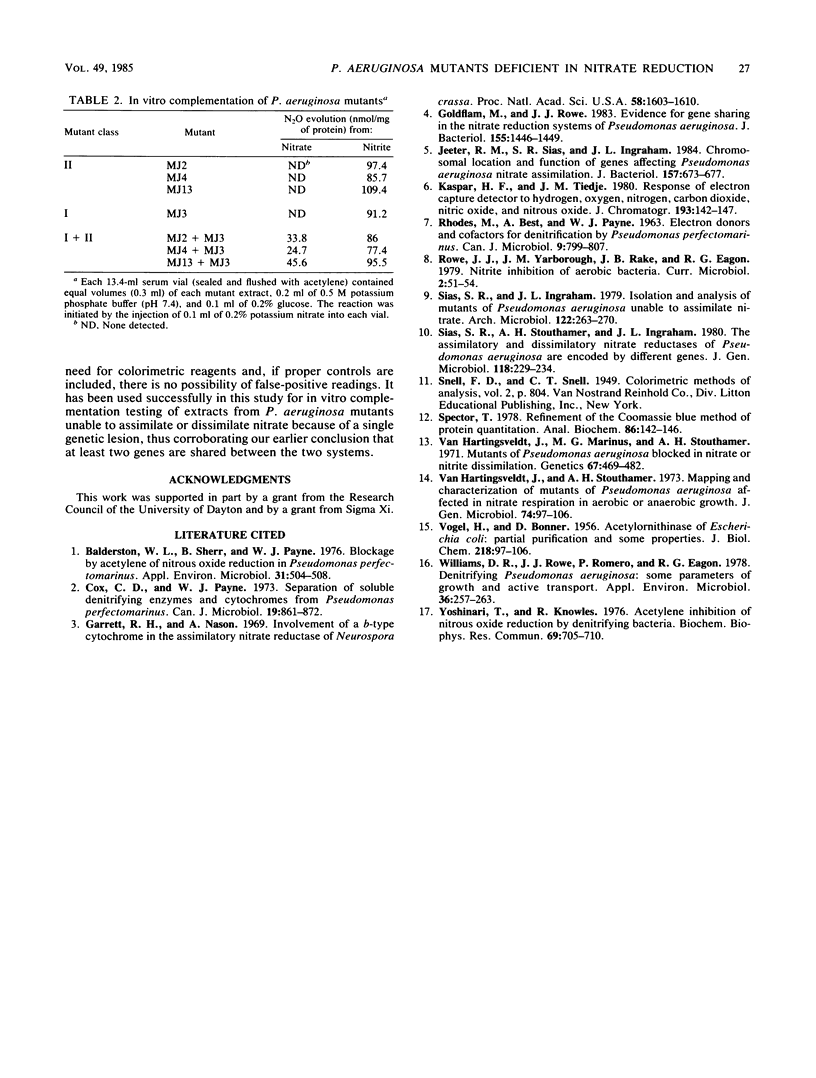
Abstract
An electron capture gas-chromatographic technique was developed to continuously measure nitrate (NO3-) reduction during in vitro complementation tests with extracts from Pseudomonas aeruginosa mutants deficient in both assimilatory and dissimilatory nitrate reduction as a result of a single genetic mutation. The procedure involves coupling nitrate reduction to nitrous oxide (N2O) evolution via a series of reactions specific to the denitrification pathway. The assay was dependent on nitrate concentration, and optimal activity was obtained with a final concentration of 0.2% potassium nitrate. The reduction exhibited a stoichiometry of 2:1 (NO3-/N2O), and succinate was the best electron source for the reaction, followed by glucose, pyruvate, and malate. The technique can also be used for continuously monitoring nitrate reduction. The optimal nitrite concentration in the nitrite reductase assay was 0.025%. The initial complementation studies of mutant extracts demonstrated that at least two genes are shared between the two nitrate reduction pathways in P. aeruginosa.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J. Separation of soluble denitrifying enzymes and cytochromes from Pseudomonas perfectomarinus. Can J Microbiol. 1973 Jul;19(7):861–872. doi: 10.1139/m73-137. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldflam M., Rowe J. J. Evidence for gene sharing in the nitrate reduction systems of Pseudomonas aeruginosa. J Bacteriol. 1983 Sep;155(3):1446–1449. doi: 10.1128/jb.155.3.1446-1449.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Sias S. R., Ingraham J. L. Chromosomal location and function of genes affecting Pseudomonas aeruginosa nitrate assimilation. J Bacteriol. 1984 Feb;157(2):673–677. doi: 10.1128/jb.157.2.673-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sias S. R., Ingraham J. L. Isolation and analysis of mutants of Pseudomonas aeruginosa unable to assimilate nitrate. Arch Microbiol. 1979 Sep;122(3):263–270. doi: 10.1007/BF00411289. [DOI] [PubMed] [Google Scholar]
- Sias S. R., Stouthamer A. H., Ingraham J. L. The assimilatory and dissimilatory nitrate reductases of Pseudomonas aeruginosa are encoded by different genes. J Gen Microbiol. 1980 May;118(1):229–234. doi: 10.1099/00221287-118-1-229. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Hartingsveldt J., Marinus M. G., Stouthamer A. H. Mutants of Pseudomonas aeruginosa bblocked in nitrate or nitrite dissimilation. Genetics. 1971 Apr;67(4):469–482. doi: 10.1093/genetics/67.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Rowe J. J., Romero P., Eagon R. G. Denitrifying Pseudomonas aeruginosa: some parameters of growth and active transport. Appl Environ Microbiol. 1978 Aug;36(2):257–263. doi: 10.1128/aem.36.2.257-263.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- van Hartingsveldt J., Stouthamer A. H. Mapping and characerization of mutants of Pseudomonas aeruginosa affected in nitrate respiration in aerobic or anaerobic growth. J Gen Microbiol. 1973 Jan;74(1):97–106. doi: 10.1099/00221287-74-1-97. [DOI] [PubMed] [Google Scholar]



