Abstract
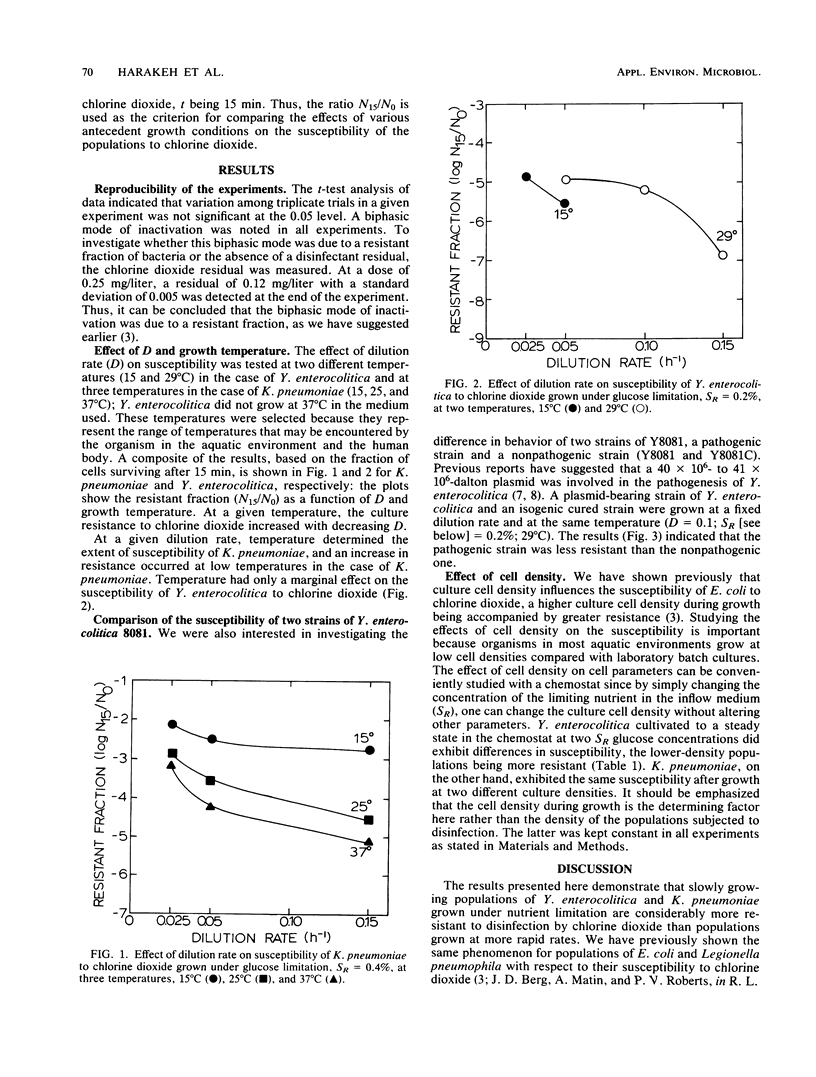
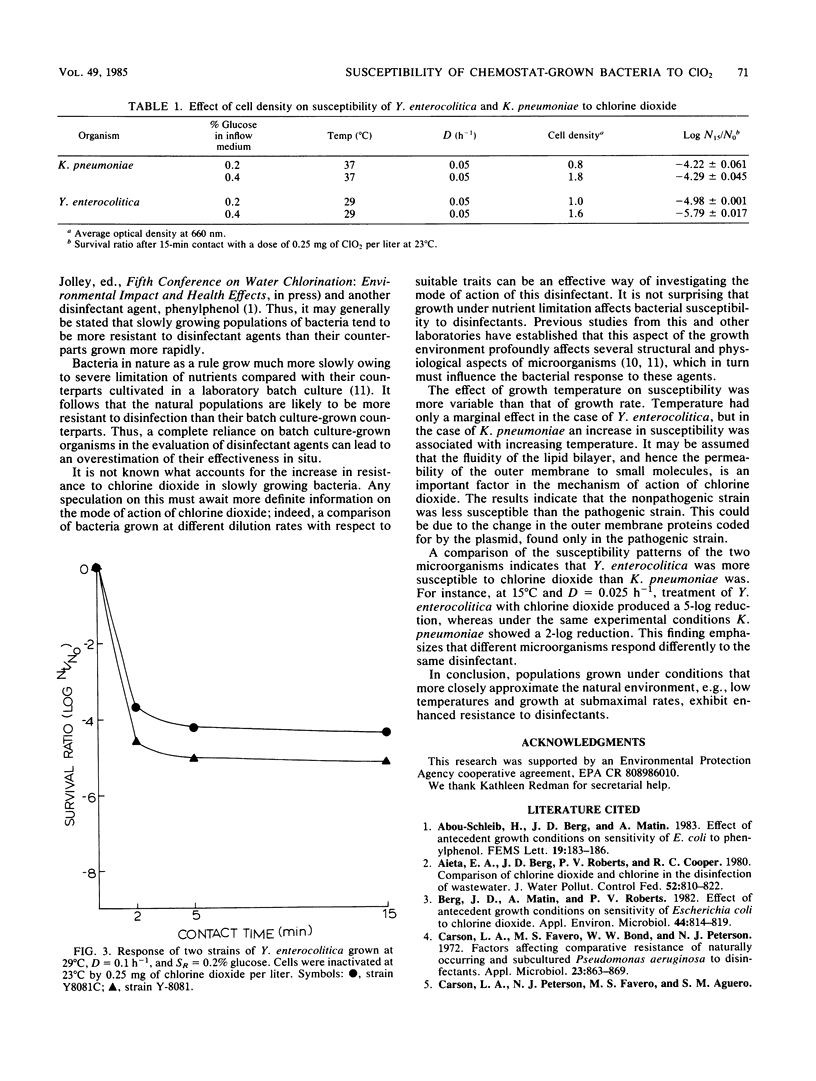
The resistance of bacteria to antimicrobial agents could be influenced by growth environment. The susceptibility of two enteric bacteria, Yersinia enterocolitica and Klebsiella pneumoniae, to chlorine dioxide was investigated. These organisms were grown in a defined medium in a chemostat and the influence of growth rate, temperature, and cell density on the susceptibility was studied. All inactivation experiments were conducted with a dose of 0.25 mg of chlorine dioxide per liter in phosphate-buffered saline at pH 7.0 and 23 degrees C. The results indicated that populations grown under conditions that more closely approximate natural aquatic environments, e.g., low temperatures and growth at submaximal rates caused by nutrient limitation, were most resistant. The conclusion from this study is that antecedent growth conditions have a profound effect on the susceptibility of bacteria to disinfectants, and it is more appropriate to use the chemostat-grown bacteria as test organisms to evaluate the efficacy of a certain disinfectant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aieta E. M., Berg J. D., Roberts P. V., Cooper R. C. Comparison of chlorine dioxide and chlorine in wastewater disinfection. J Water Pollut Control Fed. 1980 Apr;52(4):810–824. [PubMed] [Google Scholar]
- Berg J. D., Matin A., Roberts P. V. Effect of antecedent growth conditions on sensitivity of Escherichia coli to chlorine dioxide. Appl Environ Microbiol. 1982 Oct;44(4):814–819. doi: 10.1128/aem.44.4.814-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Favero M. S., Bond W. W., Petersen N. J. Factors affecting comparative resistance of naturally occurring and subcultured Pseudomonas aeruginosa to disinfectants. Appl Microbiol. 1972 May;23(5):863–869. doi: 10.1128/am.23.5.863-869.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M. S., Drake C. H. Factors influencing the occurrence of high numbers of iodine-resistant bacteria in iodinated swimming pools. Appl Microbiol. 1966 Jul;14(4):627–635. doi: 10.1128/am.14.4.627-635.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C. O., Suisted J. R. The effects of temperature and growth rate on the proportion of unsaturated fatty acids in bacterial lipids. J Gen Microbiol. 1978 Jan;104(1):31–36. doi: 10.1099/00221287-104-1-31. [DOI] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


