Abstract
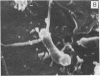
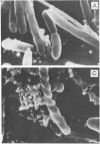
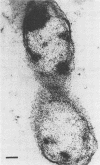
A gram-negative bacterium found to be closely associated with oysters has been isolated and characterized. The organism, designated LST, has a generation time of 106 min in Marine broth under optimal growth conditions at 25°C. During the decline phase of growth, it exhibits a morphological transition from a motile rod (ca. 1 μm in length) to an elongated, 3- to 40-μm, nonmotile, tightly coiled helix. LST synthesizes and releases a pigment in the stationary and decline phases of growth. Identified as melanin on the basis of chemical properties and UV absorbance maxima, the pigment comprises polymers of heterogeneous molecular weights, ranging from 12,000 to 120,000. The guanosine-plus-cytosine content of the LST DNA is 46%, and results of phenetic analysis and DNA-DNA hybridization indicate that this bacterium represents a new species. LST adheres to a variety of surfaces, including glass, plastics, and oyster shell, and has been shown to promote the settlement of oyster larvae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Mikami Y. Chromogenicity of Streptomyces. Appl Microbiol. 1972 Feb;23(2):402–406. doi: 10.1128/am.23.2.402-406.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurstad K., Dahle H. K. The production and some properties of the brown pigment of Aeromonas liquefaciens. Acta Vet Scand. 1972;13(2):251–259. doi: 10.1186/BF03548579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas M. R., Colwell R. R. Scanning electron microscope observation of the swarming phenomenon of Vibrio parahaemolyticus. J Bacteriol. 1982 May;150(2):956–959. doi: 10.1128/jb.150.2.956-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Weiner R. M. Photomicrography of nalidixic acid treated Hyphomicrobium neptunium: inhibition of bud formation and bud separation. Can J Microbiol. 1975 Feb;21(2):226–230. doi: 10.1139/m75-032. [DOI] [PubMed] [Google Scholar]
- Bloomfield B. J., Alexander M. Melanins and resistance of fungi to lysis. J Bacteriol. 1967 Apr;93(4):1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. A., Hinegardner R. T. Initiation of metamorphosis in laboratory cultured sea urchins. Biol Bull. 1974 Jun;146(3):335–342. doi: 10.2307/1540409. [DOI] [PubMed] [Google Scholar]
- Casida L. E., Jr Interval scanning photomicrography of microbial cell populations. Appl Microbiol. 1972 Jan;23(1):190–192. doi: 10.1128/am.23.1.190-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth H. W., Coleman J. E. Physicochemical and kinetic properties of mushroom tyrosinase. J Biol Chem. 1970 Apr 10;245(7):1613–1625. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Havenner J. A., McCardell B. A., Weiner R. M. Development of Defined, Minimal, and Complete Media for the Growth of Hyphomicrobium neptunium. Appl Environ Microbiol. 1979 Jul;38(1):18–23. doi: 10.1128/aem.38.1.18-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Holmes R. K. Isolation and characterization of melanin-producing (mel) mutants of Vibrio cholerae. Infect Immun. 1980 Mar;27(3):721–729. doi: 10.1128/iai.27.3.721-729.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield C. I., Inniss W. E. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977 Sep;23(9):1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- Nicolaus R. A., Piattelli M., Fattorusso E. The structure of melanins and melanogenesis. IV. On some natural melanins. Tetrahedron. 1964 May;20(5):1163–1172. doi: 10.1016/s0040-4020(01)98983-5. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J., Hamilton-Miller J. M. Brown- and red-pigmented Pseudomonas aeruginosa: differentiation between melanin and pyorubrin. J Med Microbiol. 1975 Feb;8(1):199–203. doi: 10.1099/00222615-8-1-199. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Hearing V. J., Kwon-Chung K. J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H., Murthy V. V. Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch Biochem Biophys. 1974 Jan;160(1):73–82. doi: 10.1016/s0003-9861(74)80010-x. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F., Harris E. B. Oxidation of phenolic compounds by Mycobacterium leprae and inhibition of phenolase by substrate analogues and copper chelators. J Bacteriol. 1968 Jun;95(6):2051–2053. doi: 10.1128/jb.95.6.2051-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Swan G. A. Structure, chemistry, and biosynthesis of the melanins. Fortschr Chem Org Naturst. 1974;31(0):521–582. doi: 10.1007/978-3-7091-7094-6_9. [DOI] [PubMed] [Google Scholar]
- Waite J. H., Tanzer M. L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science. 1981 May 29;212(4498):1038–1040. doi: 10.1126/science.212.4498.1038. [DOI] [PubMed] [Google Scholar]
- Weiner R. M., Voll M. J., Cook T. M. Nalidixic acid for enrichment of auxotrophs in cultures of Salmonella typhimurium. Appl Microbiol. 1974 Oct;28(4):579–581. doi: 10.1128/am.28.4.579-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUSSMAN R. A., LYON I., VICHER E. E. Melanoid pigment production in a strain of Trichophyton rubrum. J Bacteriol. 1960 Nov;80:708–713. doi: 10.1128/jb.80.5.708-713.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]