Abstract
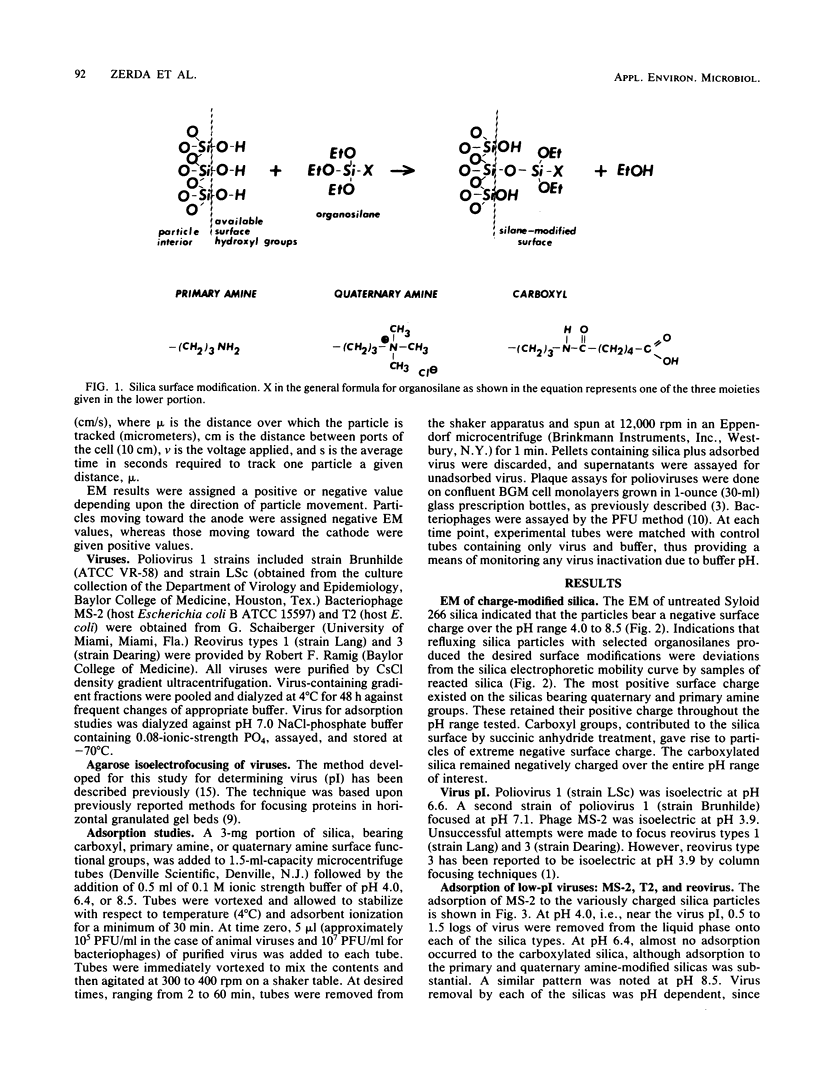
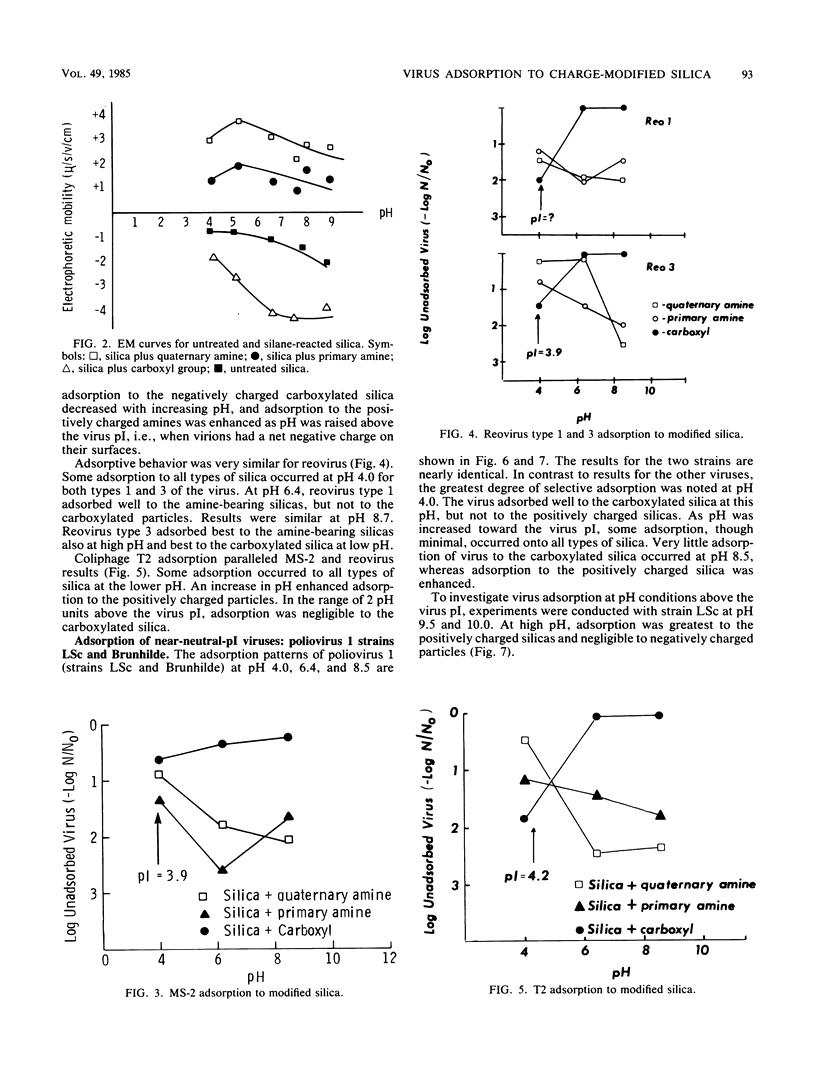
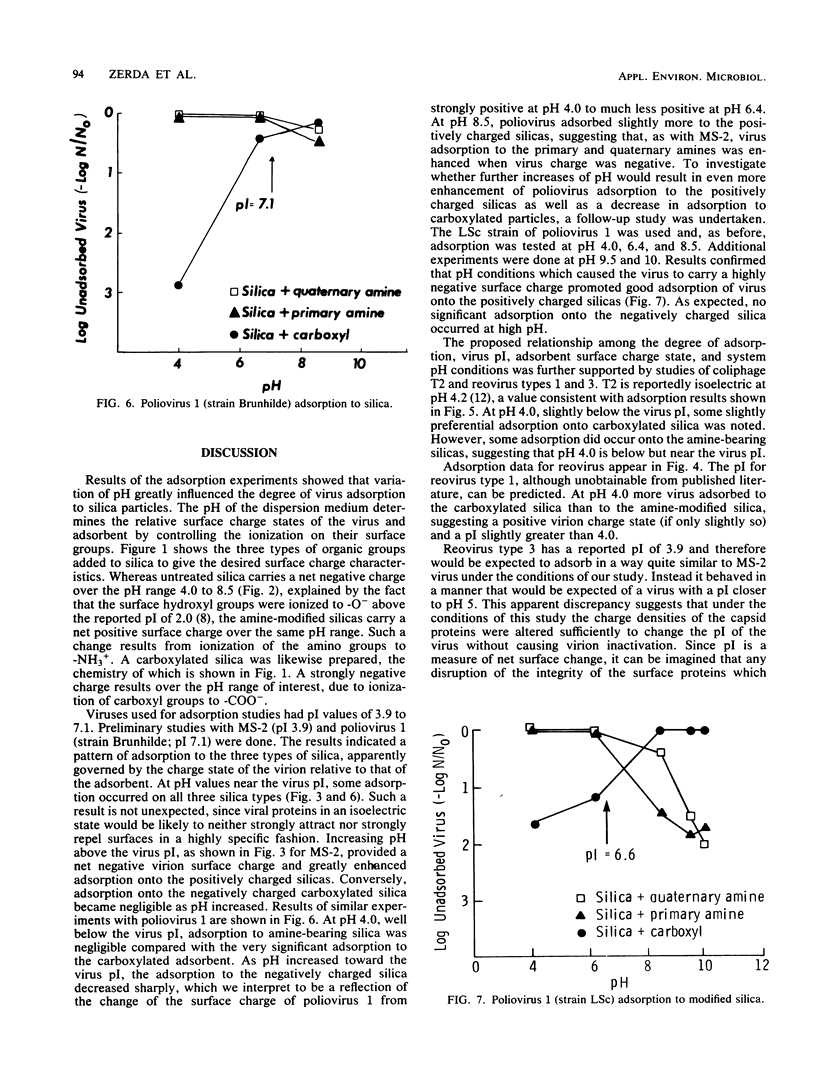
The purpose of this study was to provide a clearer understanding of virus adsorption, focusing specifically on the role of electrostatic interactions between virus particles and adsorbent surfaces. The adsorption of poliovirus 1, reovirus types 1 and 3, and coliphages MS-2 and T2 to colloidal silica synthetically modified to carry either positive or negative surface charge was evaluated. Adsorption experiments were performed by combining virus and silica in 0.1-ionic-strength buffers of pH 4.0, 6.4, and 8.5. Samples agitated for specified adsorption periods were centrifuged to pellet adsorbent particles plus adsorbed virus, and the supernatants were assayed for unadsorbed virus. All viruses adsorbed exclusively to negatively charged silica at pH values below their isoelectric points, i.e., under conditions favoring a positive surface charge on the virions. Conversely, all viruses adsorbed exclusively to positively charged silica at pH values above their isoelectric points, i.e., where virus surface charge is negative. Viruses in near-isoelectric state adsorbed to all types of silica, albeit to a lesser degree.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KETLER A., HAMPARIAN V. V., HILLEMAN M. R. Characterization and classification of ECHO 28-rhinovirus-coryzavirus agents. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:821–831. doi: 10.3181/00379727-110-27662. [DOI] [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P., Melnick J. L. Virus movement in soil columns flooded with secondary sewage effluent. Appl Environ Microbiol. 1976 Oct;32(4):520–526. doi: 10.1128/aem.32.4.520-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. I. Thin-layer isoelectric focusing of proteins. Biochim Biophys Acta. 1973 Feb 21;295(2):412–428. doi: 10.1016/0005-2795(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Salo R. J., Cliver D. O. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch Virol. 1976;52(4):269–282. doi: 10.1007/BF01315616. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerda K. S., Gerba C. P. Agarose isoelectrofocusing of intact virions. J Virol Methods. 1984 Aug;9(1):1–6. doi: 10.1016/0166-0934(84)90077-6. [DOI] [PubMed] [Google Scholar]


