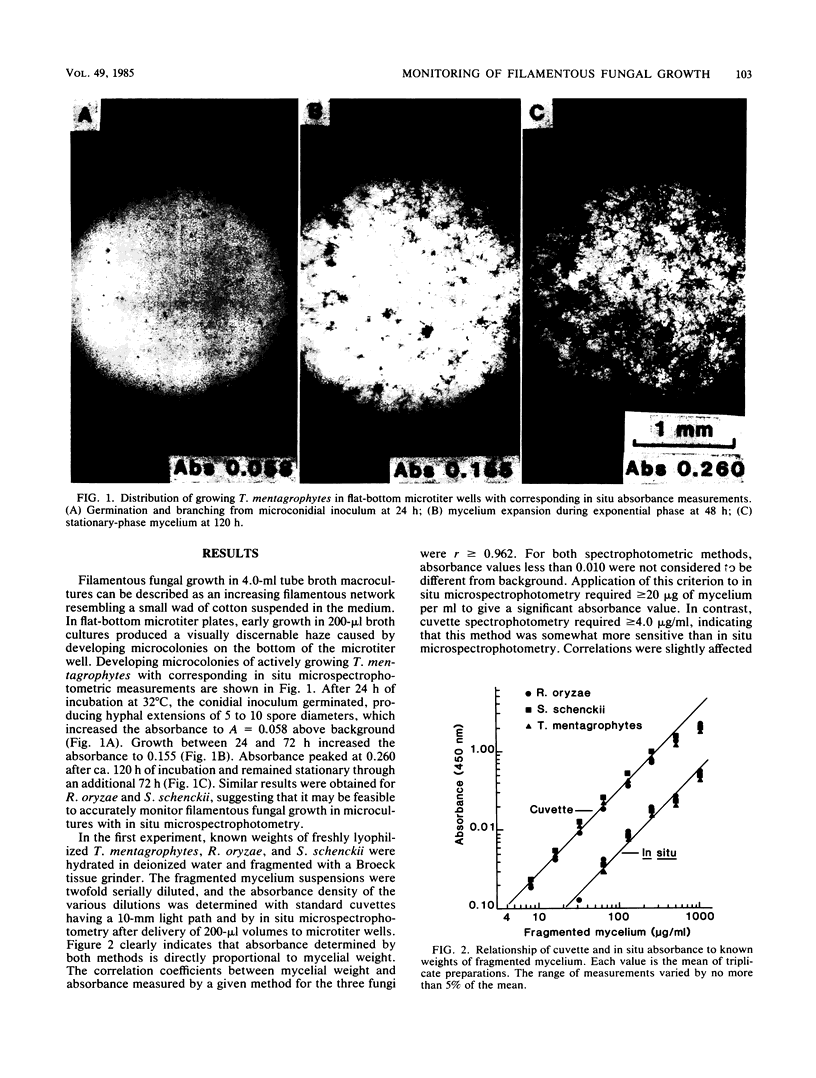
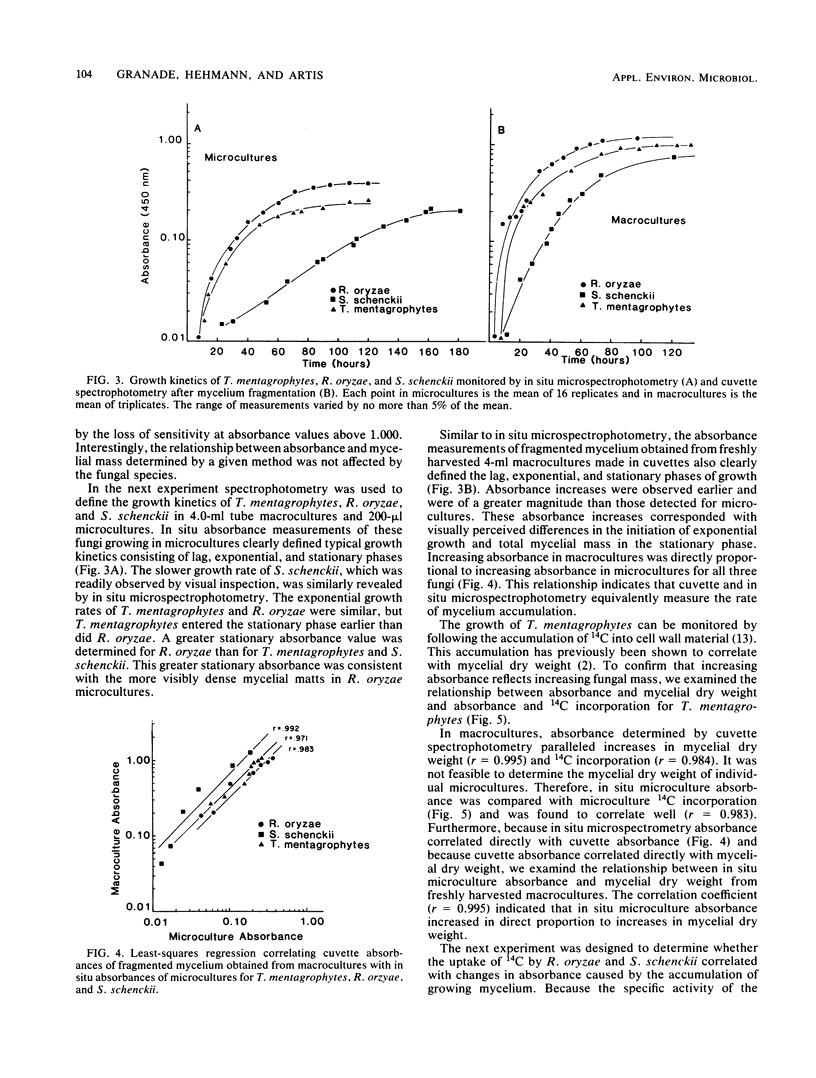
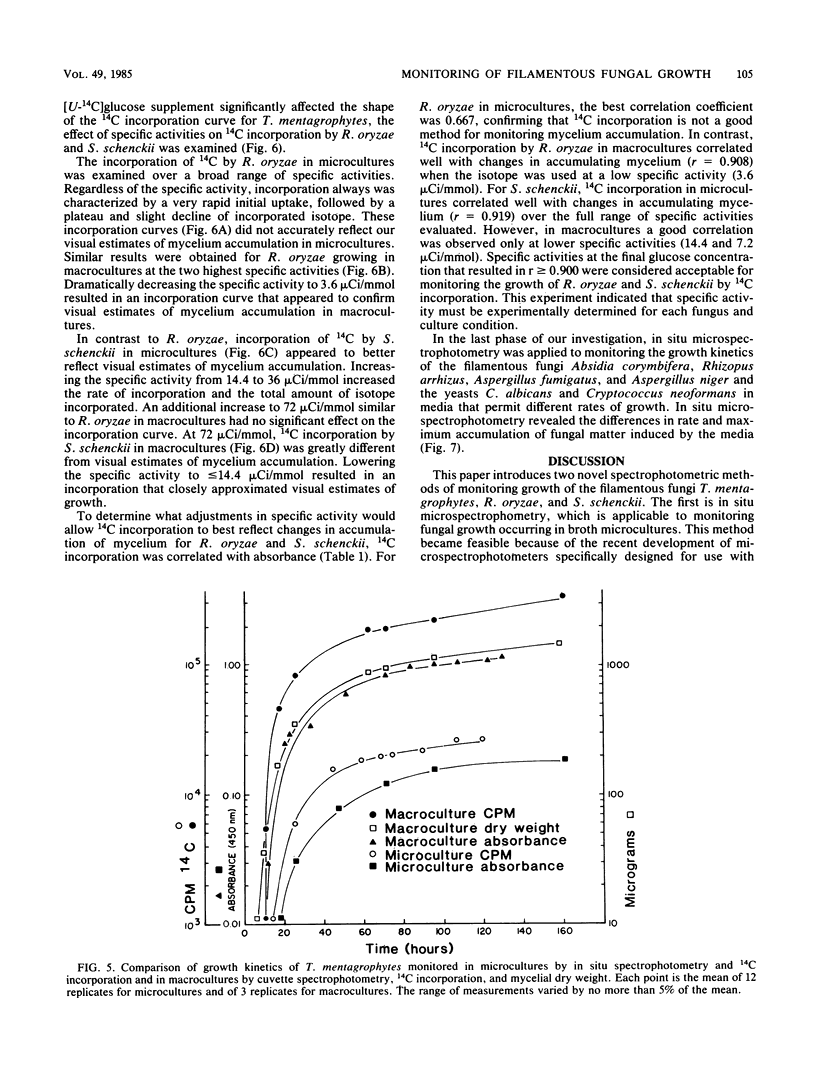
Abstract
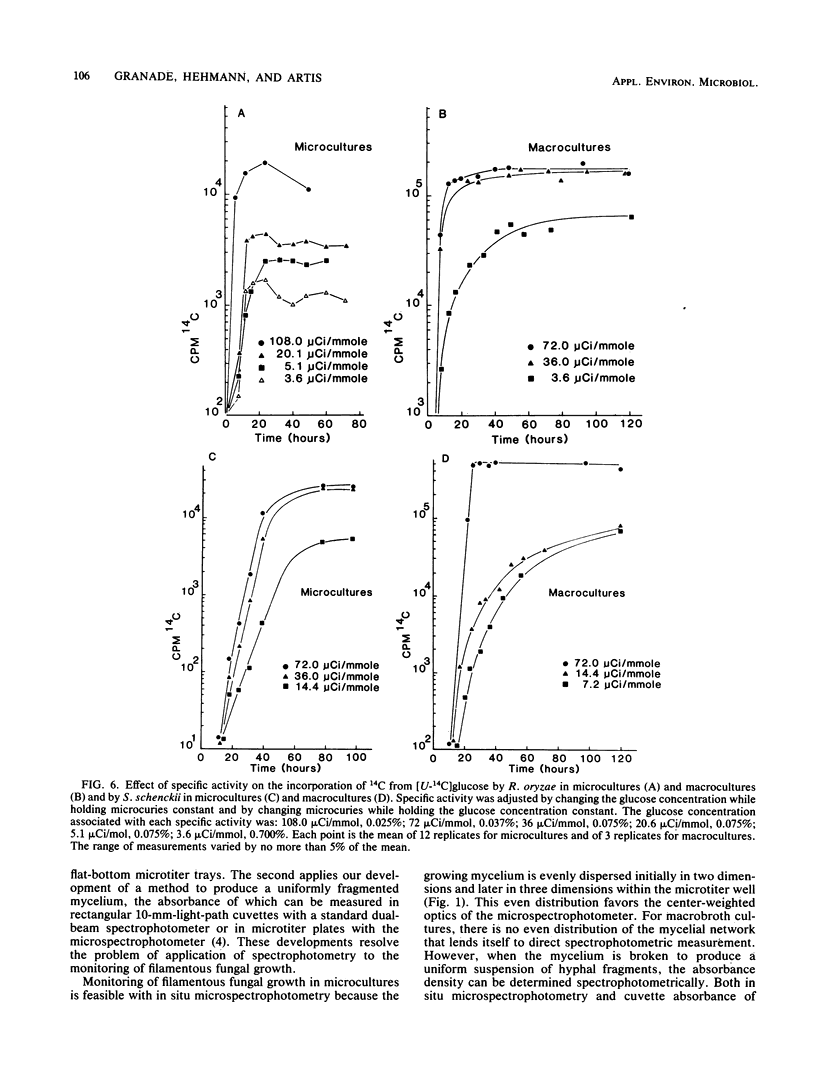
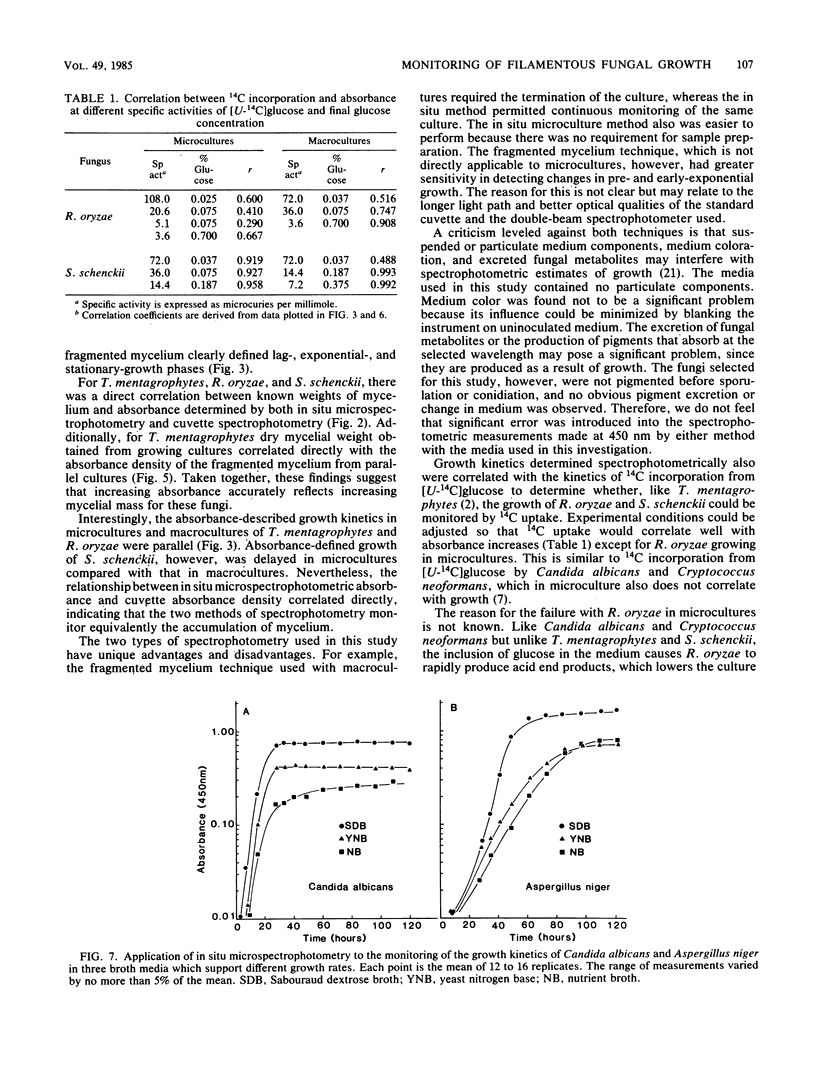
Monitoring of filamentous fungal growth by spectrophotometry is generally considered not feasible. This report describes the monitoring of growth of the filamentous fungi Trichophyton mentagrophytes, Rhizopus oryzae, and Sporothrix schenckii in broth by two new spectrophotometric methods and by 14C incorporation from [U-14C]glucose. Microcultures (200 microliter) were prepared in 96-well, flat-bottom microtiter trays, and macrocultures (4 ml) were prepared in glass vials proportionally scaled up from microcultures. Mycelium accumulation in microcultures was measured without terminating the cultures by in situ microspectrophotometry. Accumulation in macrocultures was monitored by uniformly fragmenting the mycelium with a Broeck tissue grinder and by measuring absorbance density in plastic cuvettes with a dual-beam spectrophotometer. Absorbance measurements were found to increase linearly with mycelial weight. In situ absorbance correlated with absorbance density of fragmented mycelium, indicating that both methods monitored growth equivalently. Both defined lag-, exponential-, and stationary-growth phases. Increases in 14C incorporation, absorbance, and mycelial dry weight were kinetically identical for macrocultures and microcultures of T. mentagrophytes. For R. oryzae and S. schenckii, with the exception of R. oryzae growing in microcultures, incorporation of 14C also defined lag, exponential, and stationary growth after selection of the appropriate isotope-specific activity. This incorporation correlated directly with absorbance. We conclude that in situ microspectrophotometry, fragmented mycelium absorbance density, and, to a lesser extent, 14C incorporation can be used to effectively monitor filamentous fungal growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artis W. M., Bolles R. E., Jones H. E. Filamentous fungal growth assay: correlation between [U-14C] glucose uptake and dry weight determinations. Sabouraudia. 1979 Sep;17(3):323–329. doi: 10.1080/00362177985380471. [DOI] [PubMed] [Google Scholar]
- Artis W. M., Patrusky E., Rastinejad F., Duncan R. L., Jr Fungistatic mechanism of human transferrin for Rhizopus oryzae and Trichophyton mentagrophytes: alternative to simple iron deprivation. Infect Immun. 1983 Sep;41(3):1269–1278. doi: 10.1128/iai.41.3.1269-1278.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granade T. C., Artis W. M. Antimycotic susceptibility testing of dermatophytes in microcultures with a standardized fragmented mycelial inoculum. Antimicrob Agents Chemother. 1980 Apr;17(4):725–729. doi: 10.1128/aac.17.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R. E., Newell S. Y. An improved gas chromatographic method for measuring glucosamine and muramic acid concentrations. Anal Biochem. 1983 Feb 1;128(2):438–445. doi: 10.1016/0003-2697(83)90398-6. [DOI] [PubMed] [Google Scholar]
- Ingham E. R., Klein D. A. Relationship between fluorescein diacetate-stained hyphae and oxygen utilization, glucose utilization, and biomass of submerged fungal batch cultures. Appl Environ Microbiol. 1982 Aug;44(2):363–370. doi: 10.1128/aem.44.2.363-370.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualman S. J., Jones H. E., Artis W. M. An automated radiometric microassay of fungal growth: quantitation of growth of T. mentagrophytes. Sabouraudia. 1976 Nov;14(3):287–297. doi: 10.1080/00362177685190431. [DOI] [PubMed] [Google Scholar]
- Reinhardt J. H., Allen A. M., Gunnison D., Akers W. A. Experimental human Trichophyton mentagrophytes infections. J Invest Dermatol. 1974 Nov;63(5):419–422. doi: 10.1111/1523-1747.ep12676579. [DOI] [PubMed] [Google Scholar]
- Shiraishi A., Arai T. Antifungal activity of transferrin. Sabouraudia. 1979 Mar;17(1):79–83. doi: 10.1080/00362177985380101. [DOI] [PubMed] [Google Scholar]
- Trinci A. P. A study of the kinetics of hyphal extension and branch initiation of fungal mycelia. J Gen Microbiol. 1974 Mar;81(1):225–236. doi: 10.1099/00221287-81-1-225. [DOI] [PubMed] [Google Scholar]
- Zabriskie D. W., Humphrey A. E. Estimation of fermentation biomass concentration by measuring culture fluorescence. Appl Environ Microbiol. 1978 Feb;35(2):337–343. doi: 10.1128/aem.35.2.337-343.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]