Abstract
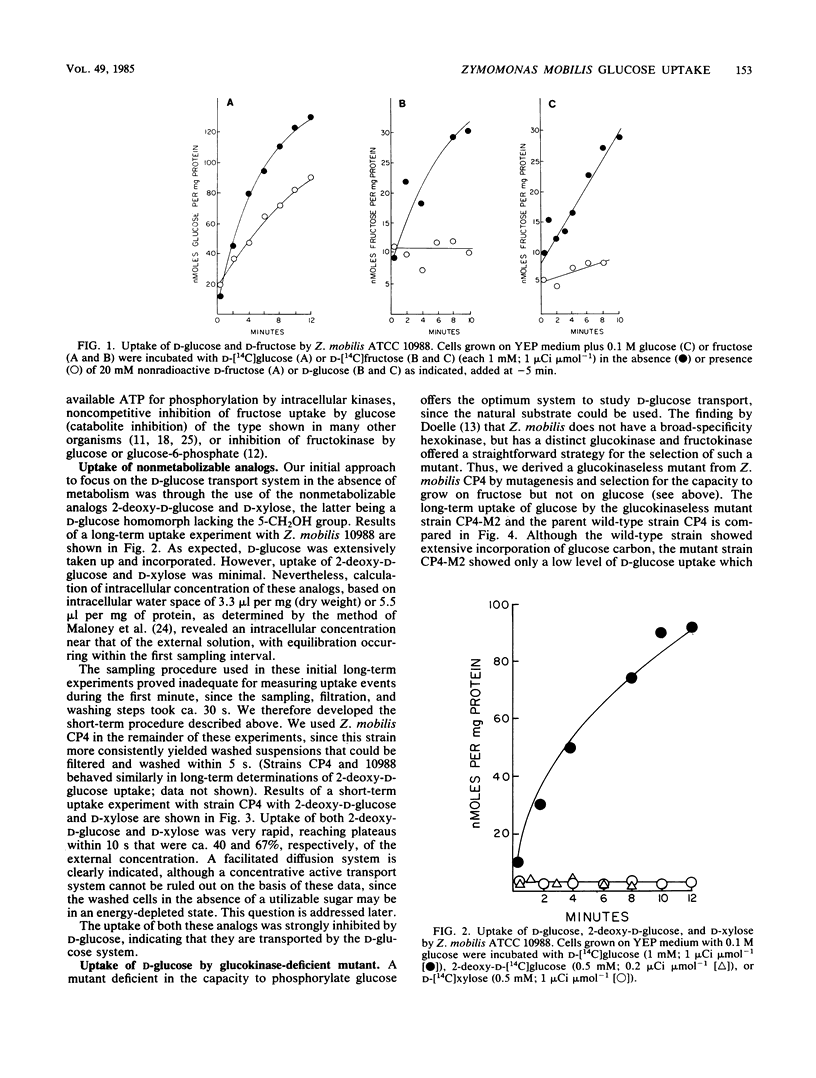
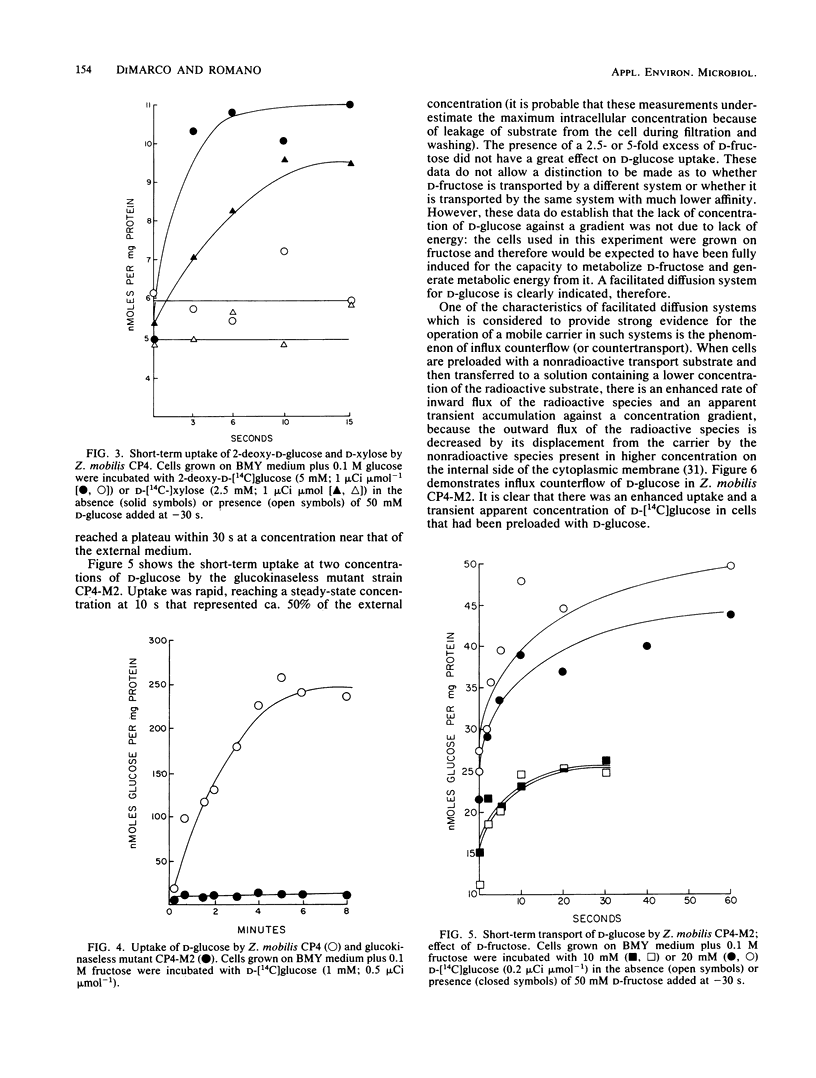
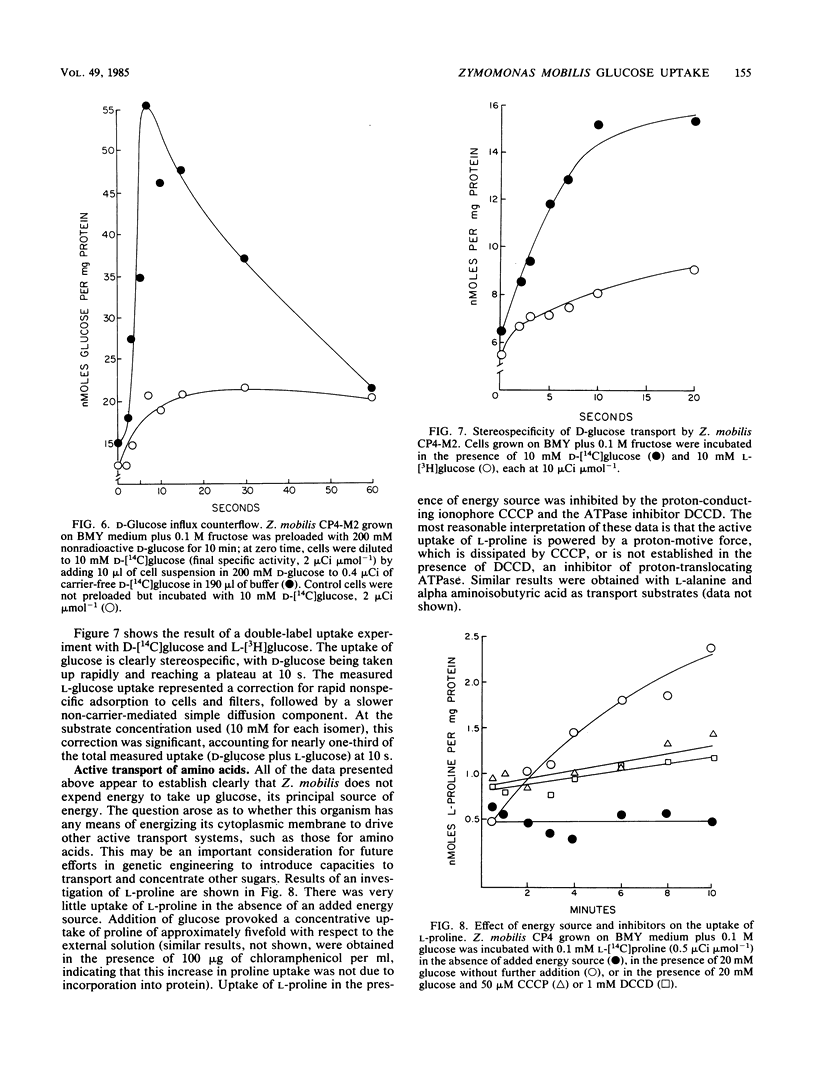
The properties of the d-glucose transport system of Zymomonas mobilis were determined by measuring the uptake of nonmetabolizable analogs (2-deoxy-d-glucose and d-xylose) by wild-type cells and the uptake of d-glucose itself by a mutant lacking glucokinase. d-Glucose was transported by a constitutive, stereospecific, carrier-mediated facilitated diffusion system, whereby its intracellular concentration quickly reached a plateau close to but not above the external concentration. d-Xylose was transported by the d-glucose system, as evidenced by inhibition of its uptake by d-glucose. d-Fructose was not an efficient competitive inhibitor of d-glucose uptake, indicating that it has a low affinity for the d-glucose transport system. The apparent Km of d-glucose transport was in the range of 5 to 15 mM, with a Vmax of 200 to 300 nmol min−1 mg of protein−1. The Km of Z. mobilis glucokinase (0.25 to 0.4 mM) was 1 order of magnitude lower than the Km for d-glucose transport, although the Vmax values for transport and phosphorylation were similar. Thus, glucose transport cannot be expected to be rate limiting at concentrations of extracellular glucose normally used in fermentation processes, which greatly exceed the Km for the transport system. The low-affinity, high-velocity, nonconcentrative system for d-glucose transport described here is consistent with the natural occurrence of Z. mobilis in high-sugar environments and with the capacity of Z. mobilis for rapid conversion of glucose to metabolic products with low energetic yield.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Relationship between sugar structure and competition for the sugar transport system in Bakers' yeast. J Bacteriol. 1968 Feb;95(2):603–611. doi: 10.1128/jb.95.2.603-611.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Ribbons D. W., Rees D. A. Sucrose utilization by Zymomonas mobilis: formation of a levan. Biochem J. 1966 Mar;98(3):804–812. doi: 10.1042/bj0980804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W. Energies of activation and uncoupled growth in Streptococcus faecalis and Zymomonas mobilis. J Bacteriol. 1967 Nov;94(5):1459–1463. doi: 10.1128/jb.94.5.1459-1463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., DEMOSS R. D. Anaerobic dissimilation of C14-labeled glucose and fructose by Pseudomonas lindneri. J Biol Chem. 1954 Apr;207(2):689–694. [PubMed] [Google Scholar]
- Kornberg H. L. Fine control of sugar uptake by Escherichia coli. Symp Soc Exp Biol. 1973;27:175–193. [PubMed] [Google Scholar]
- Kotyk A. Properties of the sugar carrier in baker's yeast. II. Specificity of transport. Folia Microbiol (Praha) 1967;12(2):121–131. doi: 10.1007/BF02896872. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Delafuente G. Regulatory properties of the constitutive hexose transport in Saccharomyces cerevisiae. Mol Cell Biochem. 1974 Dec 20;5(3):161–171. doi: 10.1007/BF01731379. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Tribe D. E., Rogers P. L. R-Plasmid Transfer in Zymomonas mobilis. Appl Environ Microbiol. 1980 Jul;40(1):7–12. doi: 10.1128/aem.40.1.7-12.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Dally E. L., Yablonsky M. D., Eveleigh D. E. Comparison of plasmids in strains of Zymomonas mobilis. Plasmid. 1983 Mar;9(2):138–146. doi: 10.1016/0147-619x(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S. K., Carey V. C., All B. P., 3rd, Ingram L. O. Self-transmissible plasmid in Zymomonas mobilis carrying antibiotic resistance. Appl Environ Microbiol. 1984 Jan;47(1):198–200. doi: 10.1128/aem.47.1.198-200.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


