Abstract
Fluid intelligence (Gf) refers to the ability to reason and to solve new problems independently of previously acquired knowledge. Gf is critical for a wide variety of cognitive tasks, and it is considered one of the most important factors in learning. Moreover, Gf is closely related to professional and educational success, especially in complex and demanding environments. Although performance on tests of Gf can be improved through direct practice on the tests themselves, there is no evidence that training on any other regimen yields increased Gf in adults. Furthermore, there is a long history of research into cognitive training showing that, although performance on trained tasks can increase dramatically, transfer of this learning to other tasks remains poor. Here, we present evidence for transfer from training on a demanding working memory task to measures of Gf. This transfer results even though the trained task is entirely different from the intelligence test itself. Furthermore, we demonstrate that the extent of gain in intelligence critically depends on the amount of training: the more training, the more improvement in Gf. That is, the training effect is dosage-dependent. Thus, in contrast to many previous studies, we conclude that it is possible to improve Gf without practicing the testing tasks themselves, opening a wide range of applications.
Keywords: cognitive training, transfer, individual differences, executive processes, control processes
Fluid intelligence (Gf) is a complex human ability that allows us to adapt our thinking to a new cognitive problem or situation (1). Gf is critical for a wide variety of cognitive tasks (2), and it is considered one of the most important factors in learning. Moreover, Gf is closely related to professional and educational success (3–6), especially in complex and demanding environments (7). There is considerable agreement that Gf is robust against influences of education and socialization, and it is commonly seen as having a strong hereditary component (2, 8, 9). Gf can be compromised as seen in the effects of certain manipulations that threaten one's group membership (10). But can Gf be improved by any means?
In the domain of psychopharmacology, although there is a market for so-called “smart drugs,” there is no study showing evidence for a drug-related increase in Gf in healthy adults although there are certain psychomotor stimulants and D2 dopamine-receptor agonists that have effects on isolated cognitive processes (11, 12). Beyond the psychopharmacology, there is a growing interest in whether computer and video games may increase IQ. But in contrast to suggestive advertisements, there is no empirical evidence that computer games enhance anything beyond task-specific performance (13, 14) and selective visuospatial attention (15).
Of course, one can easily increase performance in tests of Gf by simply practicing the tests themselves (16). However, it has been demonstrated that practice on these tests decreases their novelty and with that the underlying Gf-processes (5) so that the predictive value of the tests for other tasks disappears (17). These findings are compatible with a long history of research on cognitive training in psychological and educational science showing that, although performance on trained tasks can increase dramatically, transfer of this learning to other tasks or domains remains shockingly rare (18–21).
Despite the many failures to find transfer in any domain, the sheer importance of identifying tasks that can lead to improvement in other tasks recommends continued investigation of transfer effects. With respect to Gf, the issue is whether one can identify a task that shares many of the features and processes of Gf tasks, but that is still different enough from the Gf tasks themselves to avoid mere practice effects. A recently proposed hypothesis by Halford et al. (22) might serve as a useful framework for the design of a transfer study in which one would like to improve Gf by means of a working memory task. Their claim is that working memory and intelligence share a common capacity constraint. This capacity constraint can be expressed either by the number of items that can be held in working memory or by the number of interrelationships among elements in a reasoning task. The reason for a common capacity limitation is assumed to lie in the common demand for attention when temporary binding processes are taking place to form representations in reasoning tasks (22). Other authors came to a related conclusion, stating that Gf and working memory are primarily related through attentional control processes (23, 24). Furthermore, Carpenter et al. (1) have proposed that the ability to derive abstract relations and to maintain a large set of possible goals in working memory accounts for individual differences in typical tasks that measure Gf.
The underlying neural circuitries provide additional evidence for the shared variance between working memory and Gf in that both seem to rely on similar neural networks, most consistently located in lateral prefrontal and parietal cortices (23, 25). Therefore, it seems plausible that the training of a certain neural circuit might lead to transfer on other tasks that engage similar or at least overlapping neural circuits.
Although working memory capacity and Gf may share common variance, they are far from being isomorphic (26, 27). That is, there are factors other than working memory capacity contributing to individual differences in Gf. Nevertheless, we propose that, with a training intervention that strongly relies on binding processes and attentional control, it may be possible to produce transfer effects from a trained task to a reasoning task in which performance relies to a large extent on the same processes. There are, indeed, some studies showing that training on working memory with young healthy adults may lead to effects that go beyond a specific training effect (28, 29). So, it seems that there is some potential for transfer after training on working memory.
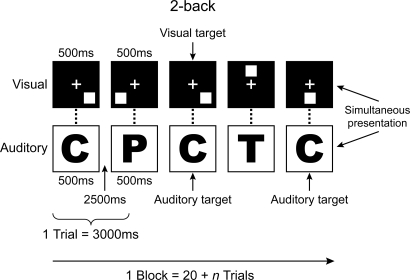
To investigate whether training on working memory leads to transfer to Gf, we conducted four individual experiments all using a newly developed training paradigm consisting of a very demanding working memory task, illustrated in Fig. 1. In this task, participants saw two series of stimuli that were synchronously presented at the rate of 3 s per stimulus. One string of stimuli consisted of single letters whereas the other consisted of individual spatial locations marked on a screen. The task was to decide for each string whether the current stimulus matched the one that was presented n items back in the series. The value of n varied from one block of trials to another, with adjustments made continuously for each participant based on performance. As performance improved, n incremented by one item; as it worsened, n decremented by one item. Thus, the task changed adaptively so that it always remained demanding, and this demand was tailored to individual participants. This form of training engaged processes required for the management of two simultaneous tasks; it engaged executive processes required for each task; and it discouraged the development of task-specific strategies and the engagement of automatic processes because of the variation in n and because of the inclusion of two different classes of stimuli.
Fig. 1.
The n-back task that was used as the training task, illustrated for a 2-back condition. The letters were presented auditorily at the same rate as the spatial material was presented visually.
The aim of the training intervention was the investigation of the effects of training on the working memory task and its impact on Gf. We accomplished this investigation by pretesting participants on a measure of Gf and then posttesting them on another form of this measure. Because we hypothesized that any alteration of the processing system would take some time to be effective, an important difference among the four experiments was the number of training sessions between pre- and posttests, ranging from 8 to 19 sessions. To control for mere retest effects, the performance of the trained groups was compared with control groups who were also assessed on Gf, but who were not trained between the two testing sessions.
Results
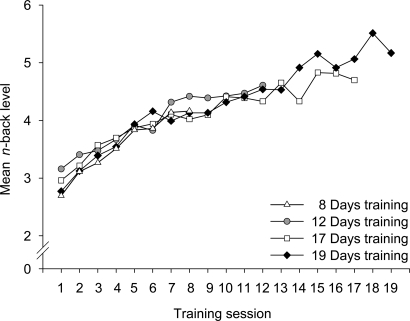
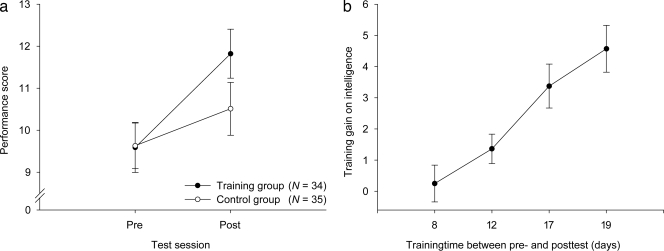
Analyses of the training functions revealed that all four training groups improved in their performance on the working memory task comparably (Fig. 2).§ What interests us most, however, is the dramatic improvement on the test of Gf in the trained groups (Fig. 3a). Although the gain in the control groups was also significant, presumably because of retest effects (t(34) = 2.08; P < 0.05; Cohen's d = 0.25), the improvement in the groups that received the apparent benefit of training was substantially superior (t(33) = 5.53; P < 0.001; Cohen's d = 0.65), which was confirmed by the significant group × test-session interaction (F(1,67) = 5.27; P < 0.05; ηp2 = 0.07). A subsequent analysis of the gain scores (posttest minus pretest) as a function of training time (8, 12, 17, or 19 days) showed that transfer to fluid intelligence varied as a function of training time (F(3,30) = 9.25; P < 0.001; ηp2 = 0.48; Fig. 3b). Analyses of covariance (ANCOVA) with the factor group (trained vs. control), the posttest scores as the dependent variable, and the pretest scores as the covariate revealed a trend for group differences after 12 days (F(1,19) = 1.93; P = 0.09; ηp2 = 0.09), and statistically significant group differences after 17 (F(1,13) = 4.65; P < 0.05; ηp2 = 0.26), and 19 training days (F(1,12) = 4.53; P < 0.05; ηp2 = 0.27). Post hoc analyses (Gabriel's procedure; two-tailed) for the training group revealed significant differences between the following groups: 8 vs. 17 days (P < 0.01); 8 vs. 19 days (P < 0.001); and 12 vs. 19 days (P < 0.01). There was a trend for a difference between 12 and 17 days (P = 0.06). These analyses indicate that the gain in fluid intelligence was responsive to the dosage of training.
Fig. 2.
Performance increase in the trained task shown separately for each training group. For each session, the mean level of n achieved by the participants is presented. The level of n depends on the participants' performance.
Fig. 3.
Transfer effects. (a) Mean values and corresponding standard errors of the fluid intelligence test scores for the control and the trained groups, collapsed over training time. (b) The gain scores (posttest minus pretest scores) of the intelligence improvement plotted for training group as a function of training time. Error bars represent standard errors.
It is important to note that the gain in Gf is strictly training-related and not due to preexisting individual differences in intelligence or working memory. There was an effect of training (F(1,68) = 6.38; P < 0.05; ηp2 = 0.09) irrespective of initial Gf as shown by dividing the sample into high and low performers in Gf at pretest (by median split). However, there was also a main effect of performance group (F(1,68) = 4.56; P < 0.05; ηp2 = 0.07), showing that participants with initially lower Gf generally showed even larger gains in Gf. There was no significant performance group by training-gain interaction (F < 1).
Further, the gain in Gf was not dependent on initial working memory capacity as assessed either by pretest performance in a digit-span task (F(1,68) < 0.3) or a reading-span task (F(1,52) < 0.1; note that reading span was not assessed in the 8-day group). Therefore, our cognitive training proved to be useful for all participants and had no adverse effects for participants with high initial working memory capacity.
Additional analyses showed that there was training-related transfer on the digit-span task (group × session interaction: F(1,67) = 14.08; P < 0.001; ηp2 = 0.17), but not on the reading span task, in which both groups improved equally in the posttest session. However, the training-time-dependent gain in Gf remained intact after controlling for the gain in working memory capacity as measured by a performance increase in both the digit-span task (ANCOVA: F(3,29) = 8.93; P < 0.001; ηp2 = 0.48) and the reading-span task (ANCOVA: F(2,22) = 6.19; P < 0.01; ηp2 = 0.36). Furthermore, the training-time-dependent gain in Gf also remained intact after controlling for the averaged n-back level reached in the last training session (ANCOVA: F(3,29) = 7.80; P < 0.001 ηp2 = 0.45).
In sum, these data indicate that the transfer effect on Gf scores goes beyond an increase in working memory capacity alone. We discuss this point in more detail below.
Discussion
We set the stage for our observed transfer effect by establishing that there is an impressive learning curve for the training task in all four experiments as expressed in comparable monotonically increasing training functions across all of the training intervals. These training results indicate that participants were challenged and motivated to improve their performance even after a training time as long as 4 weeks.
Having established a training effect, we then documented the striking result of a training-related gain in Gf, a finding that has not been reported previously. How can such a transfer effect arise?
Operationally, we believe that the gain in Gf emerges because of the inherent properties of the training task. The adaptive character of the training leads to continual engagement of executive processes while only minimally allowing the development of automatic processes and task-specific strategies. As such, it engages g-related processes (5, 17). Furthermore, the particular working memory task we used, the “dual n-back” task, engages multiple executive processes, including ones required to inhibit irrelevant items, ones required to monitor ongoing performance, ones required to manage two tasks simultaneously, and ones required to update representations in memory. In addition, it engages binding processes between the items (i.e., squares in spatial positions and consonants) and their temporal context (30, 31).
Examining the transfer task in terms of the processes involved, there is evidence that it shares some important features with the training task, which might help to explain the transfer from the training task to the Gf measures. First of all, it has been argued that the strong relationship between working memory and Gf primarily results from the involvement of attentional control being essential for both skills (22). By this account, one reason for having obtained transfer between working memory and measures of Gf is that our training procedure may have facilitated the ability to control attention. This ability would come about because the constant updating of memory representations with the presentation of each new stimulus requires the engagement of mechanisms to shift attention. Also, our training task discourages the development of simple task-specific strategies that can proceed in the absence of controlled allocation of attention.
Carpenter et al. (1) have proposed that the ability to abstract relations and to maintain a large set of possible goals in working memory accounts for individual differences in tasks such as the Raven's Advanced Progressive Matrices test, and therefore in Gf. This ability to maintain multiple goals in working memory seems especially crucial in speeded Gf tasks because one can speed performance by maintaining more goals in mind at once to foster selection among representations. Therefore, after training working memory, participants should be able to come up with more correct solutions within the given time limit of our speeded version of the Gf task.
However, our additional analyses show that there is more to transfer than mere improvement in working memory capacity in that the increase in Gf was not directly related to either preexisting individual differences in working memory capacity or to the gain in working memory capacity as measured by simple or complex spans, or even, by the specific training effect itself.
Therefore, it seems that the training-related gain on Gf goes beyond what sheer capacity measures even if working memory capacity is relevant to both classes of tasks. Of course, tasks that measure Gf are picking up other cognitive skills as well, and perhaps the training is having an effect on these skills even if measures of capacity are not sensitive to them. One example might be multiple-task management skills. Our dual n-back task requires the ability to manage two n-back tasks simultaneously, and it may be this skill that is common to tasks that measure Gf. Our measures of working memory capacity, by contrast, index capacity only for simpler working memory tasks that are not so demanding of multiple-task management skills. So, sheer working memory capacity alone may be an important component of measures of Gf, but beyond this capacity, there may be other skills not measured by simpler working memory tasks that are engaged by our training task and that train skills needed in measures of Gf. It may still be the case that training on the dual n-back task promotes development of these non-capacity skills. A line of evidence consistent with this idea shows that individual differences in both working memory span and in n-back tasks are related to individual differences in Gf (23, 25, 32).
The finding that the transfer to Gf remained even after taking the specific training effect into account seems to be counterintuitive, especially because the specific training effect is also related to training time. The reason for this capacity might be that participants with a very high level of n at the end of the training period may have developed very task specific strategies, which obviously boosts n-back performance, but may prevent transfer because these strategies remain too task-specific (5, 20). The averaged n-back level in the last session is therefore not critical to predicting a gain in Gf; rather, it seems that working at the capacity limit promotes transfer to Gf.
Of particular importance is the finding that preexisting interindividual differences in Gf as measured in the pretest are not related to the training-related gain in Gf. This finding indicates that the effect of training is not restricted to participants within a certain range of cognitive abilities. Both initial low-Gf as well as initial high-Gf participants profit from training similarly. Still, although the interaction was not reliable, we remain cautious about this result because numerically the low-Gf participants showed somewhat larger gains than the high-Gf participants. Of course, this result may be accounted for by regression to the mean, but it may also be that the training was of truly greater benefit to lower Gf participants, if not reliably so in our study.
The dose-responsive gain in Gf indicates that the training benefit is not a threshold phenomenon. The constraints of our experiments do not permit us to know how much longer we could have continued training before failing to realize any further gains in Gf. This dose responsiveness is an important issue for further study because the exact plot of gain with training could have important practical implications for those interested in training fluid intelligence. Our study also does not permit us to know how long the training gain persists; longitudinal studies will be required to address that issue.
These limitations notwithstanding, our findings are of general significance because they provide evidence for enhancement of fluid intelligence by cognitive training different from training the test itself. The finding that cognitive training can improve Gf is a landmark result because this form of intelligence has been claimed to be largely immutable. Instead of regarding Gf as an immutable trait, our data provide evidence that, with appropriate training, there is potential to improve Gf. Moreover, we provide evidence that the amount of Gf-gain critically depends on the amount of training time. Considering the fundamental importance of Gf in everyday life and its predictive power for a large variety of intellectual tasks and professional success, we believe that our findings may be highly relevant to applications in education.
Materials and Methods
Participants and Procedure.
For this study, we conducted four individual experiments involving a total of 70 healthy young participants (36 female; mean age, 25.6 years of age; SD, 3.3) recruited from the University of Bern community, 35 of whom performed working-memory training in four different training settings (one of the participants failed to complete the required training sessions and was thus discarded from the data analysis, resulting in a final N of 34). These training groups were matched to four control groups who did not have training (n = 35). The crucial difference among the four training settings was the number of training sessions between pre- and posttests, ranging from 8 to 19 sessions (i.e., 8 days (n = 16), 12 days (n = 22), 17 days (n = 16), and 19 days (n = 15)), with the control groups receiving the pre- and posttesting at the same intervals as the trained groups. In each training setting, participants trained daily, except for the weekends. The posttest took place at least 1 day after the last training session, with the largest interval being 2 days.
Materials.
Training task.
For the training task, we used the same material as described by Jaeggi et al. (33), which was a dual n-back task where squares at eight different locations were presented sequentially on a computer screen at a rate of 3 s (stimulus length, 500 ms; interstimulus interval, 2,500 ms). Simultaneously with the presentation of the squares, one of eight consonants was presented sequentially through headphones. A response was required whenever one of the presented stimuli matched the one presented n positions back in the sequence. The value of n was the same for both streams of stimuli. There were six auditory and six visual targets per block (four appearing in only one modality, and two appearing in both modalities simultaneously), and their positions were determined randomly. Participants made responses manually by pressing on the letter “A” of a standard keyboard with their left index finger for visual targets, and on the letter “L” with their right index finger for auditory targets. No responses were required for non-targets.
In this task, the level of difficulty was varied by changing the level of n (34), which we used to track the participants' performance. After each block, the participants' individual performance was analyzed, and in the following block, the level of n was adapted accordingly: If the participant made fewer than three mistakes per modality, the level of n increased by 1. It was decreased by 1 if more than five mistakes were made, and in all other cases, n remained unchanged.
One training session comprised 20 blocks consisting of 20 + n trials resulting in a daily training time of ≈25 min.
Transfer tasks.
We used standardized fluid intelligence tests, consisting of visual analogy problems of increasing difficulty. Each problem presents a matrix of patterns in which one pattern is missing. The task is to select the missing pattern among a set of given response alternatives. For the experiment with eight training sessions, we used the Raven's Advanced Progressive Matrices (RAPM) test, set II (35), whereas for all other experiments, we used the short version of the Bochumer Matrizen-Test (BOMAT) (36), a more difficult variant of the RAPM. For the RAPM, we used parallel forms for the pre- and posttesting by dividing the test into even and odd items (24); for the BOMAT, we used the published A and B versions. To keep the pre- and posttest sessions short enough, we allowed limited time (10 min) to complete the task, and the number of correct solutions provided in that time served as the dependent variable.¶
To control for the impact of individual differences and gain in working memory capacity, a digit-span task (38), as well as a reading span task (39), was used in the pre- and postsession. However, the reading span task was not assessed in the 8-day group.
Acknowledgments.
We thank Daniela Blaser and Oliver Markes for help with data collection and the participants for their time and effort. The preparation of this manuscript was supported by Swiss National Science Foundation Fellowships PA001-117473 (to S.M.J.) and PBBE1-117527 (to M.B.) and by grants from the National Science Foundation and the National Institute of Mental Health (to J.J.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 6791.
This article is a PNAS Direct Submission.
Note that the specific training gain was well explained by a linear function for all groups (8 days training: R2 = 0.78, F(1,134) = 473.44, P < 0.001; 12 days training: R2 = 0.81, F(1,202) = 863.59, P < 0.001; 17 days training: R2 = 0.73, F(1,287) = 769.63; 19 days training: R2 = 0.79, F(1,321) = 1230.23, P < 0.001).
Although this procedure differs from the standardized procedure, there is evidence that this timed procedure has little influence on relative standings in these tests, in that the correlation of speeded and non-speeded versions is very high (r = 0.95; ref. 37).
References
- 1.Carpenter PA, Just MA, Shell P. What one intelligence test measures: A theoretical account of the processing in the Raven Progressive Matrices Test. Psychol Rev. 1990;97:404–431. [PubMed] [Google Scholar]
- 2.Gray JR, Thompson PM. Neurobiology of intelligence: Science and ethics. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 3.Neisser U, et al. Intelligence: Knowns and unknowns. Am Psychol. 1996;51:77–101. [Google Scholar]
- 4.Rohde TE, Thompson LA. Predicting academic achievement with cognitive ability. Intelligence. 2007;35:83–92. [Google Scholar]
- 5.te Nijenhuis J, van Vianen AEM, van der Flier H. Score gains on g-loaded tests: No g. Intelligence. 2007;35:283–300. [Google Scholar]
- 6.Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. [Google Scholar]
- 7.Gottfredson LS. Why g matters: The complexity of everyday life. Intelligence. 1997;24:79–132. [Google Scholar]
- 8.Cattell RB. Theory of fluid and crystallized intelligence: A critical experiment. J Educ Psychol. 1963;54:1–22. doi: 10.1037/h0024654. [DOI] [PubMed] [Google Scholar]
- 9.Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: Theory and application to intellectual functioning. Annu Rev Psychol. 1999;50:471–507. doi: 10.1146/annurev.psych.50.1.471. [DOI] [PubMed] [Google Scholar]
- 10.Steele CM, Aronson JA. Stereotype threat does not live by Steele and Aronson (1995) alone. Am Psychol. 2004;59:47–48. doi: 10.1037/0003-066X.59.1.47. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 11.Elliott R, et al. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 12.Kimberg DY, D'Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. NeuroReport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 13.Haier RJ, et al. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Res. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- 14.Smith ME, McEvoy LK, Gevins A. Neurophysiological indices of strategy development and skill acquisition. Brain Res Cognit Brain Res. 1999;7:389–404. doi: 10.1016/s0926-6410(98)00043-3. [DOI] [PubMed] [Google Scholar]
- 15.Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 16.Bors DA, Vigneau F. The effect of practice on Raven's Advanced Progressive Matrices. Learn Individ Differ. 2003;13:291–312. [Google Scholar]
- 17.Ackerman PL. Individual differences in skill learning: An integration of psychometric and information processing perspectives. Psychol Bull. 1987;102:3–27. [Google Scholar]
- 18.Healy AF, Wohldmann EL, Sutton EM, Bourne LE., Jr Specificity effects in training and transfer of speeded responses. J Exp Psychol Learn Mem Cognit. 2006;32:534–546. doi: 10.1037/0278-7393.32.3.534. [DOI] [PubMed] [Google Scholar]
- 19.Singley MK, Anderson JR. The transfer of cognitive skill. Cambridge, MA: Harvard Univ Press; 1989. [Google Scholar]
- 20.Ericsson AK, Delaney PF. In: Working Memory and Thinking. Logie R, Gilhooly KJ, editors. Hillsdale, NJ: Erlbaum; 1998. pp. 93–114. [Google Scholar]
- 21.Chase WG, Ericsson KA. Skilled memory. In: Anderson J. R., editor. Cognitive Skills and Their Acquisition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981. pp. 141–189. [Google Scholar]
- 22.Halford GS, Cowan N, Andrews G. Separating cognitive capacity from knowledge: A new hypothesis. Trends Cognit Sci. 2007;11:236–242. doi: 10.1016/j.tics.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 24.Kane MJ, et al. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- 25.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 26.Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: The same or different constructs? Psychol Bull. 2005;131:30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cognit Sci. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 29.Westerberg H, Klingberg T. Changes in cortical activity after training of working memory: A single-subject analysis. Physiol Behav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberauer K. Binding and inhibition in working memory: Individual and age differences in short-term recognition. J Exp Psychol Gen. 2005;134:368–387. doi: 10.1037/0096-3445.134.3.368. [DOI] [PubMed] [Google Scholar]
- 32.Kane MJ, Conway AR, Miura TK, Colflesh GJ. Working memory, attention control, and the N-back task: A question of construct validity. J Exp Psychol Learn Mem Cognit. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- 33.Jaeggi SM, et al. On how high performers keep cool brains in situations of cognitive overload. Cognit Affect Behav Neurosci. 2007;7:75–89. doi: 10.3758/cabn.7.2.75. [DOI] [PubMed] [Google Scholar]
- 34.Jonides J, et al. Verbal working memory load affects regional brain activation as measured by PET. J Cognit Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 35.Raven JC. Advanced Progressive Matrices: Sets I, II. Oxford: Oxford Univ Press; 1990. [Google Scholar]
- 36.Hossiep R, Turck D, Hasella M. Bochumer Matrizentest: BOMAT–Advanced–Short Version. Göttingen: Hogrefe; 1999. [Google Scholar]
- 37.Frearson W, Eysenck HJ. Intelligence, reaction time (RT), and a new odd-man-out RT paradigm. Pers Individ Differ. 1986;7:807–818. [Google Scholar]
- 38.Tewes U. Hamburg-Wechsler-Intelligenztest für Erwachsene (HAWIE-R) Bern, Switzerland: Hans Huber; 1991. rev 1991. [Google Scholar]
- 39.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 1980;19:450–466. [Google Scholar]