Abstract
Natural killer (NK) cells regulate various immune responses by exerting cytotoxic activity or secreting cytokines. The interaction of NK cells with dendritic cells (DC) contributes to NK cell-mediated antitumor or antimicrobial responses. However, the cellular and molecular mechanisms for controlling this interaction are largely unknown. Here, we show an involvement of Jagged2–Notch interaction in augmenting NK cell cytotoxicity mediated by DC. Enforced expression of Jagged2 on A20 cells (Jag2-A20 cells) suppressed their growth in vivo, which was abrogated by depleting NK cells. Moreover, Jag2-A20 cells exerted a suppression on the growth of nonmanipulated A20 cells in SCID mice in an NK-dependent manner. Consistently, coinoculation of A20 cells with DC overexpressing Jagged2 (Jag2-DC) suppressed the growth of A20 cells in mice. Stimulation of NK cells with Jagged2 directly enhanced their cytotoxicity, IFN-γ production, and proliferation. Ligation of Notch2 on NK cells enhanced their cytotoxic activity, and Jag2-DC or CpG-treated DC-mediated NK cell cytotoxicity was suppressed by a γ-secretase inhibitor. These results indicate that the Jagged2–Notch axis plays a crucial role in DC-mediated NK cell cytotoxicity. Furthermore, manipulation of this interaction may provide an approach to induce potent tumor immunity or to inhibit certain autoimmune diseases caused by NK cell activation.
Keywords: cytotoxicity, tumor immunity
Natural killer (NK) cells are effector cells of the innate immune system that exhibit direct cytotoxic function (1, 2). NK cell activation is determined by a delicate balance of signals delivered by inhibitory and activating receptors (1–3). Inhibitory receptors bind to MHC class I molecules and down-regulate NK cell cytotoxicity and cytokine production. Thus, missing or low self-MHC expression activates NK cells and initiates target cell lysis (2, 3). Activating receptors are Ig-like or lectin-like molecules that initiate signaling cascades similar to those in T cells. Signaling through activating receptors can overcome inhibitory signaling when the ligands for activating receptors are abundant (3).
Recent studies have revealed that NK cells interact with dendritic cells (DC) (4, 5) and that this interaction can mediate cytotoxic T cell differentiation and NK cell activation/priming (6). For instance, DC-mediated NK cell activation is crucial for antiviral immunity (5). However, the underlying molecular and cellular mechanisms to control DC-mediated NK cell activation are poorly understood.
Notch is a transmembrane receptor activated by the interaction with Notch ligands. Mammals possess four Notch receptors and five Notch ligands. Notch ligands are categorized by two families, Delta (1, 3, and 4) and Jagged (1 and 2) (7). The interaction between a ligand-receptor pair cleaves the extracellular and transmembrane domains of Notch, resulting in the translocation of the intracellular domain of Notch to the nucleus. The interaction of the intracellular domain of Notch with RBP-J allows the formation of transcriptional DNA–protein complexes, leading to specific gene expression. Notch signaling generally controls cell fate choice, cell survival, and activation in many cell types. Recent studies have demonstrated that Notch signaling controls both the development and activation of T cells (7–10). Furthermore, two papers reported that stimulation of hematopoietic stem cells by Jagged1 or Jagged2 promotes NK cell differentiation (11–12). However, whether Jagged controls mature NK cell activation or functional differentiation in vivo requires further investigation.
In this report, we addressed whether or not one of the Notch ligands, Jagged2, controls NK cell responses in vivo. We found that stimulation of NK cells by Jagged2 enhanced the antitumor cytolytic activity of NK cells in vivo and in vitro. In addition, Jagged2 expressed on DC augmented NK cell function through interaction with Notch. These results suggest that robust Notch signaling by enhanced Jagged2 expression may provide a useful strategy to eradicate tumor cells. Conversely, the inhibition of Jagged2–Notch interaction might help cure certain autoimmune diseases caused by the overactivation of NK cells.
Results
Overexpression of Jagged2 on A20 Cells Accelerated A20 Rejection in BALB/c Mice.
We first investigated the role of Jagged2 in NK cell activation by overexpressing Jagged2 on A20 cells. A20 is a BALB/c-derived B cell lymphoma cell line that expresses high levels of MHC class II, low levels of MHC class I, Jagged1 and Delta1, and no Jagged2 or Delta4 (Fig. 1a). We transfected Jagged2 (Jag2-A20 cells) or a control vector (c-A20 cells) into A20 cells, and ≈75% of Jag2-A20 cells expressed Jagged2 after zeocin selection (Fig. 1b). The expression levels of NKG2D ligands and MHC class I on Jag2-A20 cells were comparable with those of c-A20 cells (Fig. 1c).
Fig. 1.
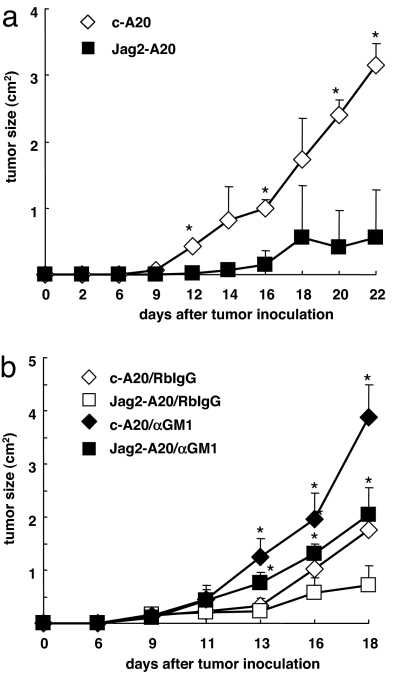
Overexpression of Jagged2 on A20 cells accelerates A20 rejection. (a) A20 cells were stained with biotin-conjugated anti-Jagged1, Jagged2, Delta1, or Delta4, followed by PE-streptavidin or FITC-conjugated anti-MHC class I (Dd) or anti-MHC class II (solid line) and expression intensity was compared with PE-streptavidin- or control Ig-stained cells (shaded histogram). (b) A20 cells were transfected with a full-length murine Jagged2-encoding vector (Jag2-A20) or control vector (c-A20). The expression of Jagged2 on Jag2-A20 or c-A20 was tested by staining cells with biotin-conjugated anti-Jagged2 antibody, followed by streptavidin-PE (solid line). Cells stained with streptavidin-PE alone were used as a control (shaded histogram). (c) The expression of NKG2D ligands or MHC class I on Jag2-A20 (thick line) or c-A20 (thin line) was tested by staining cells with NKG2D-Fc followed by FITC-conjugated anti-human IgG or FITC-conjugated anti-Dd antibody, respectively. Cells stained with streptavidin-PE or isotype control alone were used as a control (shaded histogram). (d) Jag2-A20 (filled squares) or c-A20 cells (open diamonds) were injected s.c. into BALB/c mice, and tumor size was monitored. The tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). These results are representatives of five independent experiments. (e) [3H]thymidine incorporation in c-A20 (open) or Jag2-A20 (filled) cells in cultured medium over an 8-hour period was measured. Data are shown as the mean ± SD.
To examine the effect of Jagged2 on antitumor immunity, both cell lines were inoculated s.c. in naïve BALB/c mice, and tumor sizes were monitored (Fig. 1d). The growth of c-A20 cells was detectable 19 days after inoculation, and c-A20 cells continued to grow in BALB/c mice (Fig. 1d). In contrast, Jag2-A20 cells were detectable 28 days after inoculation, and the growth of tumors was greatly suppressed compared with that of c-A20 cells (Fig. 1d). NK cells infiltrated more in Jag2-A20 than c-A20 tumors at 10 days after inoculation [supporting information (SI) Fig. S1]. These results suggest that overexpression of Jagged2 on A20 cells enhances the immune-mediated rejection of A20 cells.
To rule out the possibility that Jag2-A20 cells have acquired an intrinsic slow-proliferation phenotype, we compared the proliferation of Jag2-A20 cells and c-A20 cells in vitro. [3H]thymidine incorporation was quite similar in both cell lines under three different cell concentration conditions (Fig. 1e). Taken together, these results eliminated the possibility that the overexpressed Jagged2 directly slowed down the proliferation of A20 cells, suggesting that the retarded growth of Jag2-A20 in mice might be a consequence of augmented immune surveillance.
Growth of Jag2-A20 Cells Is Suppressed Even in SCID Mice, and NK Cells Are Involved in Jagged2-Mediated Tumor Suppression.
We next examined whether mature T or B cells caused the reduced growth of Jag2-A20 cells by inoculating Jag2-A20 cells into T cell- and B cell-deficient SCID mice. The c-A20 cells grew in SCID mice similarly in BALB/c mice (Fig. 2a). In contrast, the Jag2-A20 tumor size was much smaller in SCID mice compared with that of c-A20 cells, which indicates that T and B cells are not major players in suppressing the growth of Jag2-A20 cells (Fig. 2a). Collectively, these results suggest a role for NK cells in suppressing Jag2-A20 tumor growth. Thus, we next examined the tumor growth of Jag2-A20 cells in NK-depleted SCID mice that had been treated with anti-asialo-GM1 mAb (Fig. 2b). In fact, this treatment specifically restored the in vivo growth of Jag2-A20 cells (Fig. 2b). It is of note that the depletion of NK cells augmented the tumor growth of c-A20 cells in SCID mice, indicating that NK activation occurred to a moderate degree upon inoculation of c-A20 cells, although the activation was not strong enough to reject A20 cells. Additionally, the c-A20 tumor size was larger than that of Jag2-A20 after NK cell depletion (Fig. 2b), suggesting the presence of an NK cell-independent aspect of the Jagged2-mediated suppression in this context. Taken together, these results suggest that the overexpression of Jagged2 on A20 cells enhanced the antitumor response of NK cells against A20 cells.
Fig. 2.
NK cells are responsible for the rejection of Jag2-A20 cells. (a) Jag2-A20 (filled squares) or c-A20 (open diamonds) cells were injected s.c. into SCID mice (n = 5), and tumor size was monitored. The tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). These results are representatives of five independent experiments. (b) SCID mice injected with Jag2-A20 (squares) or c-A20 (diamonds) cells were treated with 200 μg of anti-asialo-GM1 (filled) or control rabbit (open) antibody (day −1, day 2, day 5, day 8; n = 5). The tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05) between anti-asialo-GM1-treated and control rabbit antibody-treated Jag2-A20-inoculated mice or between anti-asialo-GM1-treated and control rabbit antibody-treated c-A20-inoculated mice. These results are representatives of four independent experiments.
Enhanced Cytotoxic Activity of NK Cells from BALB/c Mice Inoculated with Jag2-A20 Cells.
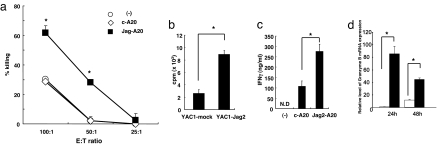
To confirm that the enhanced tumor suppression of Jag2-A20 cells reflected augmented cytotoxicity of NK cells, we purified NK cells from the spleens of c-A20- or Jag2-A20-inoculated BALB/c mice and examined them in an in vitro killing assay. The cytotoxic activity against A20 cells was markedly elevated in NK cells harvested from Jag2-A20-inoculated BALB/c mice compared with those from c-A20-inoculated or untreated mice (Fig. 3a). Therefore, these results indicate that overexpression of Jagged2 in A20 cells enhanced the killing activity of NK cells in vivo.
Fig. 3.
Enhanced cytotoxic activity of NK cells from BALB/c mice inoculated with Jag2-A20 cells. (a) c-A20 or Jag2-A20 cells (2 × 107 per mouse) were injected i.p. in BALB/c mice. NK cells were purified from spleens 4 days after injection and were cultured with 51Cr-labeled A20 cells for 5 h. Cytotoxic activity of NK cells from unimmunized (open circles), c-A20 injected (open diamonds) or Jag2-A20 injected (filled squares) mice against A20 cells were evaluated by measuring 51Cr release from A20 cells. These results are representatives of three independent experiments. The data are shown as the mean ± SD. *, statistical significance (P < 0.05). (b) Purified NK cells were stimulated with YAC-1 cells transfected with control or Jagged2-encoding vectors for 2 days. The [3H]thymidine incorporation for the final 8 h was counted. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). (c) Purified NK cells were stimulated with A20 cells transfected with control or Jagged2-encoding vectors for 3 days. The concentration of IFN-γ in the supernatant was measured by ELISA. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). N.D., not detected. (d) Purified NK cells were stimulated with OP9 cells transduced with control (open symbols) or Jagged2-encoding vectors (filled symbols) for 24 or 48 h. The expression of granzyme B in NK cells was measured by real-time PCR. All data were normalized against the G3PDH mRNA levels and expressed as fold increases relative to control values ± SD obtained from naïve NK cells. Data are shown as the mean ± SD of four samples. *, statistical significance (P < 0.05).
We next tested whether Jagged2 augments the proliferation, IFN-γ production and expression of effector molecules by NK cells. We isolated DX5+ NK cells from BALB/c mice and incubated them with YAC-1 cells transfected with a control vector or a Jagged2-encoding vector (Fig. 3b), with c-A20 or Jag2-A20 cells (Fig. 3c), or with OP9 cells transfected with a control vector or a Jagged2-encoding vector (Fig. 3d). The presence of Jagged2 on YAC-1, A20, or OP9 cells apparently enhanced the proliferation, IFN-γ secretion, or granzyme B transcription of NK cells, respectively (Fig. 3 b, c, and d). These results demonstrate that Jagged2 enhances NK cell functions, including cytotoxicity, proliferation and IFN-γ production.
Inoculation of Jag2-A20 Cells Enhanced the Antitumor Effect of NK Cells to Kill A20 Cells Transplanted at a Different Site.
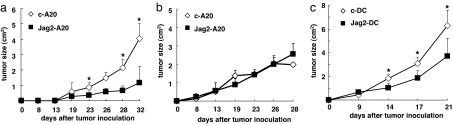
We then postulated that the exposure of NK cells to Jagged2-expressing cells could condition them to become activated killers, and these cells could subsequently eradicate bystander tumor cells that are defective for Jagged2 expression. To this end, we inoculated Jag2-A20 and A20 cells or c-A20 and A20 cells at different sites on the same SCID mouse and monitored the tumor size of the bystander A20 cells (Fig. 4a). The growth of A20 cells in SCID mice inoculated bilaterally with Jag2-A20 cells was suppressed compared with that of mice that received c-A20 cells (Fig. 4a). This effect of Jag2-A20 cells was diminished by treating SCID mice with anti-asialo-GM1 mAb, indicating that NK cells are responsible for this effect (Fig. 4b).
Fig. 4.
Inoculation of Jag2-A20 cells enables NK cells to kill A20 cells transplanted at a different site. (a) Jag2-A20 and A20 cells (closed squares) or c-A20 and A20 cells (open diamonds) were inoculated in different sites of the same SCID mouse (n = 5), and the tumor size of the A20 cells was monitored. The tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). These results are representatives of three independent experiments. (b) SCID mice (n = 5) injected with Jag2-A20 and A20 cells (filled squares) or c-A20 and A20 cells (open diamonds) were treated with 100 μg of anti-asialo-GM1 antibody (day −1, day 2, day 5, day 8; n = 5), and the tumor size of the A20 cells was monitored. Tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). These results are representatives of three independent experiments. (c) A20 cells were inoculated with DC transduced with Jagged2 (filled squares) or control vector (open diamonds) in SCID mice, and the tumor size of A20 cells was monitored. The tumor size was calculated as (axial) × (horizontal) diameter. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). These results are representatives of three independent experiments.
Transfection of Jagged2 in tumor cells is not a favorable clinical method to accomplish enhanced NK cell activity. Thus, we examined whether DC overexpressing Jagged2 (Jag2-DC) are able to augment NK cell activity. Jagged2 was transduced into bone marrow-derived DC by a retroviral vector. Then Jag2-DCs or control virus-transduced DC (c-DC) were coinjected with A20 cells, and the tumor size was monitored. The inoculation of Jag2-DC significantly suppressed the growth of A20 cells compared with c-DC (Fig. 4c). This result indicates the feasibility for the use of DC as a vehicle to enable Jagged2-mediated augmentation of NK cell activity in vivo, and opens up the possibility for therapeutic tumor intervention.
Jagged2 on DC Directly Activates NK Cells.
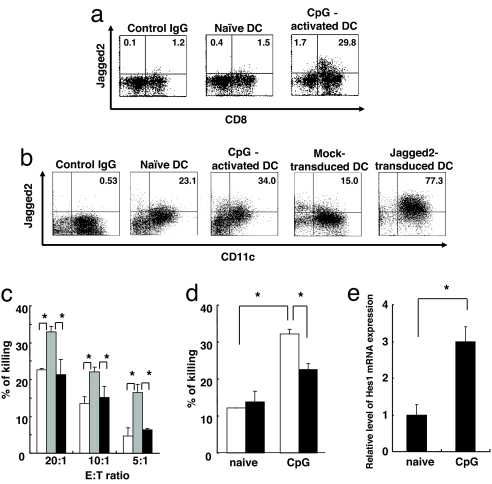
To explore the physiological roles of Jagged2 in terms of NK cell activation, we examined whether Jagged2 is involved in DC-mediated NK cell activation. We first analyzed Jagged2 expression on splenic DC. Splenic DC from untreated BALB/c mice express little Jagged2, and CpG-administration to mice up-regulated Jagged2 expression on DC (Fig. 5a). Furthermore, the expression of Jagged2 on DC was limited to the CD8α+ subset. Bone marrow-derived DC cultured in the presence of GM-CSF express Jagged2 (Fig. 5b). CpG stimulation of bone marrow-derived DC up-regulated Jagged2 expression, although the level was lower than that of DC transduced with a Jagged2-expressing retrovirus (Fig. 5b). CpG stimulation of bone marrow-derived DC also up-regulated expression of Delta1 and Jagged1 but not Delta4, compared with controls (Fig. S2).
Fig. 5.
DC-mediated NK cell activation is controlled by Notch signaling. (a) The expression of Jagged2 on splenic DC from naïve or CpG-treated mice was evaluated by flow cytometry after staining cells with PE-conjugated anti-CD11c, FITC-conjugated anti-CD8α mAb, and biotin-conjugated anti-Jagged2 mAb, followed by streptavidin-APC. Data shown are gated on CD11c+ cells. (b) The expression of Jagged2 on bone marrow-derived untreated, CpG-treated, control vector-transduced, or Jagged2-transduced DC was evaluated by flow cytometry. Cells were stained with PE-conjugated anti-CD11c mAb and biotin-conjugated anti-Jagged2 mAb, followed by streptavidin-APC. (c) NK cells from BALB/c mice were cultured with Jag2-DC in the presence (black bars) or absence (gray bars) of 10 μM GSI. The cytotoxic ability of NK cells against YAC-1 cells was measured by 51Cr release from YAC-1 cells. The activity of NK cells without Jag2-DC stimulation was used as a control (white bars). Data are shown as the mean ± SD. *, statistical significance (P < 0.05). (d) NK cells from BALB/c mice were cultured with unstimulated or CpG (2 μM)-stimulated bone marrow-derived DC for 16 h in the absence (white bars) or presence (black bars) of 10 μM GSI. NK cells were enriched and cocultured with 51Cr-labeled YAC-1 cells, and 51Cr release from YAC-1 cells was measured. Data are shown as the mean ± SD. *, statistical significance (P < 0.05). (e) NK cells from BALB/c mice were cultured with unstimulated or CpG (2 μM)-stimulated bone marrow-derived DC for 16 h. The mRNA expression of HES1 in purified NK cells was quantified by real-time PCR. The value obtained from Notch1 expression in NK cells cocultured with unstimulated DC was set as 1. Data are shown as the mean ± SD. *, statistical significance (P < 0.05).
To test whether Jagged2 expressed on DC is able to directly activate NK cells, we examined NK cell cytotoxic activity after coculture with Jag2-DC. The coculture of Jag2-DC and NK cells increased the cytotoxic activity of NK cells over that of unstimulated NK cells (Fig. 5c). We examined whether blocking Notch signaling decreases DC-mediated NK cell cytotoxicity by treating NK cells with a γ-secretase inhibitor (GSI). GSI treatment inhibited NK cell activation mediated by Jag2-DC (Fig. 5c). We next tested whether Jagged2-untransfected DC also activate NK cells in a Notch signaling-dependent manner (Fig. 5d). NK cells cocultured with CpG-activated DC exhibited increased NK cell cytotoxicity, which was suppressed by GSI treatment (Fig. 5d). Furthermore, NK cells cocultured with CpG-activated DC up-regulated the Notch target gene, HES1 (Fig. 5e). Taken together, these studies indicate that Notch signaling, possibly through Jagged2, is crucial for DC-mediated enhancement of NK cell cytotoxicity.
Notch2 Signaling Is Involved in DC-Mediated NK Cell Activation.
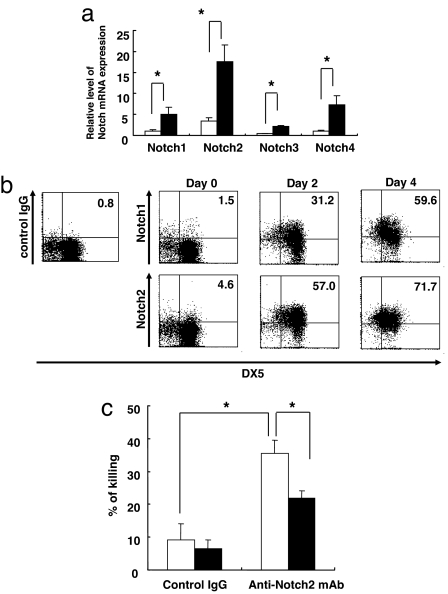
To identify the receptor that interacts with Jagged2 in our system, we tested the expression of Notch1, Notch2, Notch3, and Notch4 on unstimulated and IL-2-activated NK cells. We detected the expression of Notch1, Notch2, and Notch4 mRNA in unstimulated NK cells by RT-PCR, and we could detect the expression of Notch1–4 after IL-2 stimulation (Fig. 6a). We could also detect the expression of Notch1 and Notch2 in naïve NK cells by flow cytometry, and their expression was apparently up-regulated by IL-2 stimulation (Fig. 6b).
Fig. 6.
Notch signaling contributes to DC-mediated NK cell activation. (a). The expression of Notch1, Notch2, Notch3, or Notch4 in naïve (black bars) or IL-2-stimulated (white bars) NK cells (48 h) was evaluated by real-time PCR. All data were normalized against the G3PDH mRNA levels and expressed as fold increases relative to control values ± SD obtained from Notch1 expression in naïve NK cells. Data are shown as the mean ± SD of three samples. *, statistical significance (P < 0.05). (b) Notch1 and Notch2 expression on naïve NK cells or IL-2-stimulated DX5+ NK cells (day 0, 2, and 4) were evaluated by flow cytometry. (c) NK cells from BALB/c mice were cultured in the presence of 1 μg/ml anti-Notch2 or control antibody in the absence (white bars) or presence (black bars) of 10 μM GSI for 16 h. The cytotoxic ability of NK cells against YAC-1 cells was measured by 51Cr release from YAC-1 cells. Data are shown as the mean ± SD. *, statistical significance (P < 0.05).
We next examined whether ligation of Notch2 increases NK cell activity. NK cells were incubated with anti-Notch2 agonist antibody for 16 h, and NK cell cytotoxic activity against YAC-1 cells was measured. The ligation of Notch2 enhanced NK cell activity, and the treatment of NK cells by GSI inhibited the effect of the anti-Notch2 antibody (Fig. 6c), indicating that Notch2 is one of the functional receptors for up-regulating NK cell function.
Discussion
Notch signaling controls the development and activation of a variety of immune cells (7, 8, 10, 13, 14). Recent studies have demonstrated that Jagged and Delta are able to promote the development or activation of NK cells, at least in in vitro culture systems (11, 12, 15). However, it remains unclear whether Notch signaling controls mature NK cell activation and functional differentiation in vivo, and, if so, it should be important to know which cells affect NK cell activation through the expression of Notch ligands. In this report, we shed light on this issue by demonstrating that stimulation of NK cells with Jagged2 strengthens mature NK cell-killing activity in vivo and in vitro and that Jagged2 expressed on DC plays a crucial role in DC-mediated NK cell activation through interaction with Notch. These findings provide an insight that the modulation of Notch signaling could be a strategy to eradicate tumors or to suppress NK cell-mediated diseases.
Recent studies have provided evidence that NK cells are activated by DC and macrophages (5, 6, 16–19), although cellular and molecular mechanisms controlling DC-mediated NK cell cytotoxicity remain unclear. We have addressed this issue by examining whether Notch-signaling plays a crucial role. We observed that Jagged2 expressed on DC is able to enhance NK cell cytotoxic activity through interaction between these cells and that blockade of Notch signaling significantly attenuated the DC-mediated NK cell activation. Furthermore, stimulation of NK cells with an agonist antibody recognizing Notch2 increased NK cell-killing activity. Therefore, these results collectively indicate that Notch signaling is crucial for DC-mediated NK cell activation, possibly through Jagged2 interaction with Notch2. As for the receptor binding Jagged2 on NK cells, we cannot completely rule out the involvement of other Notch receptors, in particular Notch1, because Notch1 is highly expressed on IL-2-activated NK cells. Our present experiments revealed the contribution of Notch signaling in NK activation by using GSI, but GSI affects other signaling pathways (20). Although our present data also revealed the up-regulation of a Notch-targeted gene in NK cells by DC stimulation, it would be important to confirm the contribution of Notch in NK cell activation by using Notch gene-targeted mice. Additionally, we obtained evidence that Notch signaling directly controls the transcription of granzyme B and perforin in cytotoxic T cells (Y.M. and K.Y., unpublished observation). We also demonstrated in this study that stimulation of NK cells by Jagged2 rapidly up-regulated granzyme B mRNA. These results are in support of the notion that the direct transcriptional activation of cytolytic molecules by Notch signaling could account for the increased effector activity of NK cells. Furthermore, we demonstrated that Jagged2-mediated stimulation of NK cells promotes their proliferation in vitro. Therefore, both up-regulation of effector molecules and increased proliferation of NK cells mediated by Notch signaling would play a major role in strengthening antitumor effects.
IL-2 stimulation of NK cells up-regulates Notch1 and Notch2 expression on NK cells. Although a previous report indicated that MyD88-dependent stimulation in DC did not up-regulate Jagged2, evaluated by real-time PCR (9), we observed increased expression of Jagged2 on CpG-stimulated splenic and bone marrow-derived DC by flow cytometry, suggesting that a posttranscriptional mechanism might be involved in this expression. Therefore, TLR9-related infections or autoimmune responses may strengthen Notch-mediated NK-DC interactions. In light of recent publications demonstrating the involvement of IL-15 or NKG2D in DC-mediated NK cell activation (5, 6), it is crucial to investigate the unique physiological roles of Notch signaling in DC-mediated NK cell activation in comparison with those by other molecules.
Several previous papers demonstrated that Jagged1, Jagged2, or transient Delta1-mediated signaling stimulate NK cell development (11, 15), although it remains to be determined which Notch receptors control NK cell development. On the other hand, the present study indicates that Jagged2 directly enhances the cytotoxic activity of mature NK cells, which was inhibited by a γ-secretase inhibitor, and that the ligation of Notch2 on NK cells augmented NK cell-killing activity. Because Notch 2 is able to bind with Delta1, Delta4, or Jagged1, at least in vitro (21) (data not shown), and CpG-activated DC up-regulate Delta1, Jagged1 and Jagged2, other Notch ligands might be involved in augmenting NK cell activation. However, we think the contribution of Delta1 or Delta4 would be less likely, at least in vivo, because we did not observe an accelerated rejection of tumor cells by transfecting such ligands (data not shown). The evaluation of Jagged1 in DC-NK interactions is currently underway. An intriguing emerging question from these studies is how mature NK cells choose the correct ligand(s) for their activation, because Detla1, Delta4, and Jagged1 as well as Jagged2 are expressed on activated DC (data not shown). One possibility is that the expression pattern or timing of the Notch ligands is limited to particular cells, thus tightly controlling the interaction of NK cells with their correct ligands. Alternatively, the interaction of Notch and Notch ligands might be controlled by modifying the sugar pattern of Notch by fringe genes. The fringe-mediated addition of N-acetylglucosamine to o-fucose moieties on Notch could change the ligand sensitivity of Notch (22–24). In support of this, we have observed that NK cells express fringe genes (data not shown). Such modulation of Notch and Notch ligand interaction by fringe may contribute to the selection of the correct ligand(s) for NK cells during their development or activation.
We demonstrated here the possibility that Jagged2 overexpression on DC helps suppress the growth of tumor cells. Because vaccination by DC or by tumor antigen-loaded DC has been widely explored as a method for cancer intervention, the inclusion of Jagged2 in DC-mediated vaccination modalities may prove beneficial because this strategy leads to the activation of NK cells and tumor-specific CD8+ T cells. Furthermore, it might be possible to suppress NK cell-mediated pathogenesis, which is warranted in the context of some autoimmune diseases, by blocking the Jagged2 signaling pathway. Because Notch receptors are widely expressed on a variety of cells, whereas expression of Jagged2 is rather limited, the inhibition of Jagged2 may be preferable to that of other Notch receptors for the purpose of suppressing NK cell activity with fewer side effects.
Materials and Methods
Mice.
Six- to 8-wk-old BALB/c and SCID mice were purchased from Japan SLC, and all mice were maintained under specific pathogen-free conditions in the animal research center of the University of Tokushima. All animal studies were approved by the animal research committee of the University of Tokushima.
Transfection, Cell Culture, and Virus Infection.
The cDNA of murine Jagged2 was cloned into the pTracer/zeo vector (Fig. S3) (Invitrogen) and the pKE004 retroviral vector (8). Retroviruses were constructed by transfecting vectors into plat-E cells (25) (provided by T. Kitamura, Tokyo University, Tokyo) as reported (24). After transfection of Jagged2 into A20 or transduction of Jagged2 into YAC-1 or OP9 cells, stable A20, YAC-1, or OP9 cells expressing Jagged2 were maintained. In some experiments, A20 cells were cultured for three days and 1 μCi of [3H]thymidine (PerkinElmer Life Sciences) was added for the final 8 h. The incorporation of [3H]thymidine was counted by a liquid scintillation counter.
Bone marrow cells from BALB/c mice were collected and cultured in RPMI medium 1640 supplemented with 10% FCS and culture supernatant containing GM-CSF (provided by T. Iizuka, University of Minnesota, Minneapolis). The retroviral vector pKE004 encoding murine Jagged2 was transfected into plat-E cells. Bone marrow cells were infected with the retrovirus 0, 1, and 2 days after initial culture. The CD11c+ and GFP+ cells were sorted by the JSAN cell sorter (Bay Bioscience) 6 days after initial culture. In some experiments, bone marrow-derived CD11c+ cells were stimulated with 2 μM CpG (Hycult Biotechnology) for 24 h.
Flow Cytometry.
NK cells, bone marrow-derived DC, or A20 cells were resuspended in staining buffer (2% FCS, 0.05% sodium azide in PBS) at a cell density of 2 × 106 per milliliter. In some experiments, spleen cells from BALB/c mice injected with 50 μg of CpG or NK cells stimulated with mouse IL-2 (100 units/ml) (R&D Systems) for 3 days were resuspended in staining buffer. PE-conjugated anti-DX5 (eBiosciences), PE-conjugated anti-CD11c (eBiosciences), FITC-conjugated anti-CD8α (eBiosciences), FITC-conjugated anti-H-2Dd (BD Biosciences), FITC-conjugated anti-MHC class II, mNKG2D-Fc chimera (R&D Systems), and FITC-conjugated anti-human IgG (Chemicon) were used for cell staining. The biotin-conjugated anti-Jagged1 (HMJ1–29), Jagged2 (HMJ2–1), Delta1 (HMD1–5), Delta4 (HMD4–3), Notch1 (HMN1–12), or Notch2 (HMN2–29) mAbs were used for cell staining together with streptavidin-PE or streptavidin-APC (eBiosciences) (SI Text). Anti-Jagged1, Jagged2, Delta1, Delta4, Notch1, or Notch2 antibodies were established by immunizing Armenian hamsters with Jagged1-, Jagged2-, Delta1-, or Delta4-expressing CHO cells or Notch1-Fc or Notch2-Fc recombinant protein. The specificities for anti-Notch ligand antibodies or Notch antibodies are shown in Figs. S4 and S5, respectively. The fluorescence intensity of ≈105 cells was examined by using a FACSCalibur flow cytometer (BD Biosciences).
NK Cell Purification and Functional Analysis.
Spleen cells from unimmunized mice or mice that received 2 × 106 c-A20 or Jag2-A20 cells i.p. were collected, and IgG-positive cells were depleted by anti-mouse IgG beads (Qiagen). DX5+ cells were purified from the IgG-negative fraction by anti-DX5-coated beads (Miltenyi Biotec). The purity of the DX5+ cells was ≈90%. NK cells were stimulated with 1 μg/ml anti-Notch2 antibody for 16 h or cultured with bone marrow-derived control DC or Jagged2-transduced DC (1 × 105) for 16 h in the absence or presence of 5 μg/ml (10 μM) γ-secretase inhibitor (DAPT; Sigma). After 4 hours of coculture of NK cells with 51Cr-labeled targets at various effector/target ratios, supernatants were harvested, and their radioactivity was determined. The percentage of specific cytotoxicity (% cytotoxicity) was calculated by using the following formula: % cytotoxicity = (experimental lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100. All cytotoxicity assays were carried out in triplicate. Data are presented as the mean ± SD. Proliferation of NK cells was assessed by using purified NK cells stimulated with YAC-1 cells transfected with pKE004 or pKE004 encoding Jagged2 for 48 h. One μCi of [3H]thymidine (PerkinElmer Life Sciences) was added for the final 8 hours. The incorporation of [3H]thymidine was counted by a liquid scintillation counter. In some experiments, purified NK cells (2 × 105) were stimulated with A20 cells transfected with murine Jagged2 (2 × 105) for 3 days, and culture supernatants were collected. The concentration of IFN-γ in the supernatant was measured by ELISA according to the manufacturer's protocol (eBiosciences). To test the mRNA expression of NK cells stimulated by Jagged2, purified NK cells (2 × 105) were stimulated with OP9 cells transfected with murine Jagged2 (1 × 105) for 24 or 48 h, and then NK cells were purified by anti-DX5 antibody-coated beads.
Real-Time PCR.
RNA was isolated from NK cells by TRIzol (Invitrogen), and cDNA was generated by using Omniscript Reverse Transcription (Qiagen) according to the manufacturer's instructions. The mRNA expression levels were quantified by using the ABI 7500 Real-Time PCR System (Applied Biosystems). cDNAs were amplified with TaqMan probes using SYBR Premix Ex Taq (TaKaRa). The mRNA expression levels were normalized relative to G3PDH gene expression levels. Fold differences in mRNA levels were determined by using SDS System Software ver.1.4.0 (Applied Biosystems). Primer sequences are as follows:
Hes1 (forward: 5′-GGACAAACCAAAGACGGCCTCTGAGCACAG-3′, reverse: 5′-TGCCGGGAGCTATCTTTCTTAAGTGCATCC-3′), Granzyme B (forward: 5′-GGATATAAGGATGGTTCACC-3′, reverse: 5′-CACCTGTCCTAGAGCAATCC-3′), G3PDH (forward: 5′-TCCACCACCCTGTTGCTGTA-3′, reverse: 5′ACCACAGTCCATGCCATCAC-3′), Notch1 (forward: 5′-TGAGACTGCCAAAGTGTTGC-3′, reverse: 5′-GTGGGAGACAGAGTGGGTGT-3′), Notch2 (forward: 5′-ACAAATACTGTGCAGACCACTTCAA-3′, reverse: 5′-AGCACCACGATGATCAGGGT-3′), Notch3 (forward: 5′-CAGGCGAAAGCGAGAACAC-3′, reverse: 5′-GGCCATGTTCTTCATTCCCA-3′), Notch4 (forward: 5′-GAACGTGGATCCCCTCAAGT-3′, reverse: 5′-AGAGAGAGGGCAAGGACTCAT-3′)
Tumor Inoculation.
Mice were inoculated s.c. on the flank with 1 × 106 A20 or A20-Jag2 cells in PBS. The long and short diameters of the tumors were measured by using calipers at 2-day intervals; these two diameters were multiplied to obtain an estimate of the tumor area. Depletion of NK cells was accomplished by i.p. injections of 200 μg of polyclonal rabbit anti-asialo GM1 (Wako Chemicals) 2 days before the first tumor inoculation and then at 3-day intervals. In some experiments, 1 × 106 A20 and A20-Jag2 or c-A20 cells were injected s.c. in different sites on the same mouse, or 1 × 105 bone marrow-derived dendritic cells transduced with Jagged2 were mixed with 1 × 106 A20 cells and s.c. injected. The data are displayed as the mean ± SD of the tumor areas for each group of animals at a given time point.
Statistical Analysis.
The Mann–Whitney U test was applied to compare data from noninterval scales, such as tumor size. Normally distributed data from interval scales were analyzed by Student's t test with P < 0.05 considered to be statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Kitamura and Dr. Iizuka for providing reagents; Dr. Tagaya (National Cancer Institute, Bethesda, MD) for critically reading the manuscript; Mrs. Kinouchi for technical assistance; and Mrs. Yamakawa for secretarial assistance. This work was supported by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Knowledge Cluster Initiative from the Ministry of Education, Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709919105/DCSupplemental.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Di Santo JP. Natural killer cell developmental pathways: A question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 3.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 4.Adam C, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- 5.Andoniou CE, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 6.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 8.Maekawa Y, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 9.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 10.Tsukumo S, Yasutomo K. Notch governing mature T cell differentiation. J Immunol. 2004;173:7109–7113. doi: 10.4049/jimmunol.173.12.7109. [DOI] [PubMed] [Google Scholar]
- 11.DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521–3527. doi: 10.1182/blood-2004-11-4237. [DOI] [PubMed] [Google Scholar]
- 12.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–1447. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15:69–79. doi: 10.1016/s1044-5323(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 14.Minato Y, Yasutomo K. Regulation of acquired immune system by notch signaling. Int J Hematol. 2005;82:302–306. doi: 10.1532/IJH97.05095. [DOI] [PubMed] [Google Scholar]
- 15.Carotta S, Brady J, Wu L, Nutt SL. Transient Notch signaling induces NK cell potential in Pax5-deficient pro-B cells. Eur J Immunol. 2006;36:3294–3304. doi: 10.1002/eji.200636325. [DOI] [PubMed] [Google Scholar]
- 16.Baratin M, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci USA. 2005;102:14747–14752. doi: 10.1073/pnas.0507355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 18.Andrews DM, et al. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 19.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–383. doi: 10.1016/j.conb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu K, et al. Binding of Delta1, Jagged1, and Jagged2 to Notch2 rapidly induces cleavage, nuclear translocation, and hyperphosphorylation of Notch2. Mol Cell Biol. 2000;20:6913–6922. doi: 10.1128/mcb.20.18.6913-6922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruckner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 23.Moloney DJ, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 24.Tsukumo S, Hirose K, Maekawa Y, Kishihara K, Yasutomo K. Lunatic fringe controls T cell differentiation through modulating notch signaling. J Immunol. 2006;177:8365–8371. doi: 10.4049/jimmunol.177.12.8365. [DOI] [PubMed] [Google Scholar]
- 25.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.