Abstract
The burden of protein misfolding is believed to contribute to aging. However, the links between adaptations to conditions associated with protein misfolding and resistance to the time-dependent attrition of cellular function remain poorly understood. We report that worms lacking aip-1, a homologue of mammalian AIRAP (arsenic-inducible proteasomal 19S regulatory particle-associated protein), are not only impaired in their ability to resist exposure to arsenite but also exhibit shortened lifespan and hypersensitivity to misfolding-prone proteins under normal laboratory conditions. Mammals have a second, constitutively expressed AIRAP-like gene (AIRAPL) that also encodes a proteasome-interacting protein, which shares with AIRAP the property of enhancing peptide accessibility to the proteasome's active site. Genetic rescue experiments suggest that features common to the constitutively expressed worm AIP-1 and mammalian AIRAPL (but missing in the smaller, arsenite-inducible AIRAP) are important to lifespan extension. In worms, a single AIRAP-related protein links proteasomal adaptation to environmental stress with resistance to both proteotoxic insults and maintenance of animal life span under normal conditions.
Keywords: aging, chaperones, environmental toxins, protein degradation
Although the loss-of-function features of protein misfolding tend to manifest early and affect organismal development and reproduction, more general perturbations to protein folding homeostasis, referred to as proteotoxicity, tend to manifest later in life and are closely linked to the time- and use-dependent attrition in cellular and organismal function recognized as aging (1, 2). A relatively small number of chaperones contribute to the folding of most of the proteome. Misfolded proteins engage this chaperone network and tax its ability to cope with the physiological load of unfolded proteins (3, 4). This notion of a general protein folding homeostasis is supported by recent findings on the role of chaperones in promoting longevity in worms and other organisms. For example, overexpression of either HSP70 or HSP16 chaperones directly increases animal life span (5, 6), whereas reduced chaperone expression by heat shock factor-1 (hsf-1) knockdown shortens lifespan (7).
Chaperone induction constitutes one strand of the adaptation to the threat of proteotoxicity; it is complemented by activation of the cell's protein degradation machinery (8). Recently, specific ubiquitin ligases have been implicated in longevity; however, they are believed to exert their effects by modulating the stability of known lifespan regulators (9, 10); the direct effects of proteasome function on aging have been less well explored.
We have recently identified AIRAP as a highly conserved arsenic-inducible gene encoding a component of the proteasome's 19S regulatory cap. AIRAP and its worm homologue, aip-1, adapt the cell's protein degradation machinery to the threat of protein misfolding by arsenic (11). A second mammalian gene predicted to encode a constitutively expressed AIRAP homologue, AIRAPL, also exists and is characterized here. Worms have measurable basal levels of aip-1 mRNA (12), suggesting that a single gene incorporates features of both the arsenic-inducible AIRAP and the constitutively expressed AIRAPL. Here, we report that AIP-1 plays an important role in preserving the lifespan of worms and in buffering proteotoxicity in circumstances that are not associated with exposure to arsenic. Thus, a proteasomal adaptation that is induced by environmental stress links resistance to proteotoxic insults to the maintenance of normal animal lifespan.
Results
Worm aip-1 Is Required for Normal Lifespan and Resistance to the Consequences of Protein Misfolding.
Inactivation of aip-1 by RNAi sensitizes worms to the acute toxic effects of exposure to sodium arsenite (11, 12). However, under normal growth conditions, aip-1(RNAi) worms developed normally, were morphologically indistinguishable from reference animals, and produced a normal number of progeny (Fig. 1A).
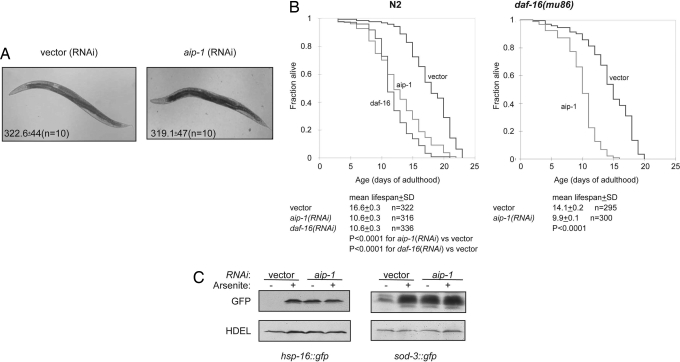
Fig. 1.
aip-1 is required for normal worm lifespan and basal stress resistance. (A) Photomicrograph of typical adult wild-type animals grown on bacteria expressing either empty vector or aip-1 dsRNA. The inset number is the mean ± SD brood size of an individual animal in each class. (B) Lifespan curves of wild-type (N2) and daf-16(mu86) mutant animals subjected to the indicated RNAi. Shown is a typical experiment repeated three times with similar results. Cumulative statistics on all three experiments are noted below. (C) Immunoblots of GFP expressed by the indicated transgenic reporter and an endogenous ER protein (detected by the anti-HDEL antibody, which serves as a loading control) in lysates of worms subjected to the indicated RNAi and exposed to sodium arsenite (3 mM) where indicated.
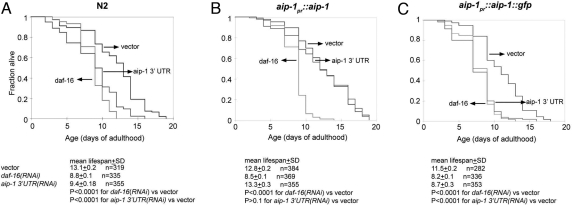
To determine whether aip-1(RNAi) affected animal lifespan, we compared the survival of cohorts of reference animals and those raised on aip-1(RNAi) from the earliest stages of larval development. The reference animals had a mean lifespan of 16.6 ± 0.3 days, whereas the aip-1(RNAi) animals had a reduced lifespan of 10.6 ± 0.3 days. The reduction in mean lifespan brought about by aip-1(RNAi) was similar in magnitude to that brought by daf-16(RNAi) (10.6 ± 0.3 days) (Fig. 1B). Although both genes are involved in the response to stress, aip-1 and daf-16 appear to function in nonoverlapping pathways because aip-1(RNAi) further decreased lifespan of daf-16(mu86) mutant animals (14.1 ± 0.2 days for mock RNAi versus 9.9 ± 0.1 days for aip-1 RNAi). Furthermore, unlike daf-16(RNAi), aip-1(RNAi) had no effect on development of the stress-resistant dauer state [supporting information (SI) Table S1].
AIRAP/AIP-1 is known to modulate the 26S proteasome in arsenite-treated cells (11), but the outcome of the lifespan studies, which were conducted in animals that had not been exposed to environmental toxins, suggests an additional role for aip-1 under normal growth conditions. To explore this possibility, we measured the effect of aip-1(RNAi) on the expression of two stress-inducible reporter genes: hsp-16pr::gfp, which reports on the activity of the heat-shock response (13), and sod-3pr::gfp, which reports on the levels of oxidative stress response (14). Both pathways were activated by arsenite in the control animals, as expected. Interestingly, in these experiments, aip-1(RNAi) markedly increased reporters' basal activity such that their levels were no longer increased further by arsenite (Fig. 1C).
The aforementioned experiments suggest that aip-1's role in counteracting proteotoxicity is not confined to arsenic exposure and might extend to other circumstances. To explore this possibility, we made use of a transgenic line constitutively expressing a fusion of YFP to a polyglutamine-containing tract of 35 residues. This Q35–YFP protein is initially soluble; however, as the animals aged, the distribution of the protein became increasingly punctate, and the detergent-insoluble fraction increased. This aging-dependent alteration, which is believed to mimic aspects of the pathogenesis of polyglutamine expansion-mediated neurodegeneration (15), is accelerated by genetic manipulations that compromise the handling of misfolded proteins (16, 17). Consistent with this idea, we found that aip-1(RNAi) of unc-54pr::Q35–yfp transgenic worms accelerated the appearance of puncta, and a similar acceleration in puncta formation was also observed in hsf-1(RNAi) animals (Fig. 2A) (16). Both aip-1(RNAi) and hsf-1(RNAi) promoted accumulation of Q35–YFP in SDS-resistant aggregates (Fig. 2B), linking the appearance of puncta to a change in the biochemical properties of the misfolding-prone fusion protein.
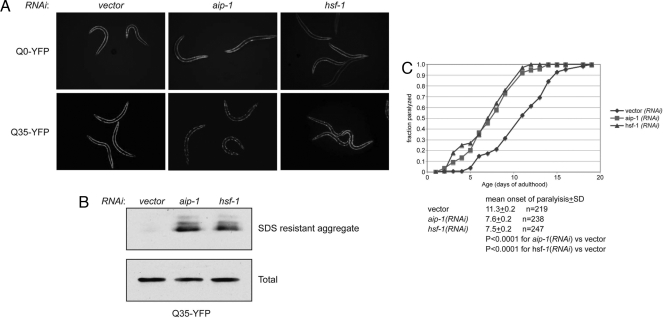
Fig. 2.
aip-1 protects against proteotoxicity. (A) Fluorescent photomicrographs of 5-day-old adult transgenic animals expressing Q0–YFP or Q35–YFP in their body wall muscle. The animals were subjected from the time of hatching to the indicated RNAi. (B) Immunoblot of the Q35–YFP fusion protein from the SDS-insoluble fraction and postnuclear supernatant (total) of 5-day-old animals shown in A. (C) Paralysis as a function of time in transgenic animals expressing an amyloidogenic peptide derived from the human Alzheimer precursor protein in their body wall muscle. Where indicated, the animals were subjected to RNAi. Shown is a typical experiment repeated three times with similar results. Cumulative statistics on all three experiments are shown.
aip-1's role in resisting proteotoxicity was explored in a second assay in which an amyloidogenic peptide derived from the Alzheimer disease precursor protein (Aβ1–42) was targeted to the body wall muscle cells, resulting in age-dependent paralysis (18, 19). aip-1(RNAi) accelerated the mean onset of paralysis from 11.3 ± 0.2 days in mock RNAi to 7.6 ± 0.2 days in aip-1(RNAi) animals. A similar effect was observed in hsf-1(RNAi) (mean onset of paralysis, 7.5 ± 0.2 days) (20) (Fig. 2C). These observations indicate that in addition to its established role in protecting against the consequences of arsenic, aip-1(RNAi) also compromises protein folding homeostasis under basal conditions, attenuates the ability of worms to cope with toxic, misfolding-prone proteins, and reduces lifespan.
A Mammalian Paralogue, AIRAP-Like (AIRAPL), Shares Features with AIP-1 and AIRAP.
Mammals have two AIP-1 homologues: the previously characterized arsenite-inducible AIRAP (encoded by ZFAND2a) and a second predicted protein that we named AIRAPL (encoded by ZFAND2b) (Fig. 3A). Northern blots showed that AIRAPL mRNA is constitutively expressed in cultured mouse fibroblasts (data not shown) and broadly represented in EST collections derived from diverse mouse and human tissues. Constitutive expression of AIRAPL was also noted in an immunoblot of cultured cell lysate (Fig. 3B).
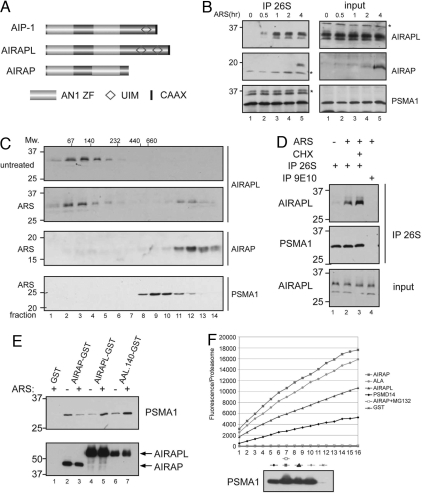
Fig. 3.
Mammalian AIRAPL association with proteasomes. (A) Cartoon depicting the domain organization of worm AIP-1 and its mammalian homologues AIRAP and AIRAPL. (B) Immunoblot of endogenous AIRAP, AIRAPL, and the 20S proteasome subunit PSMA1 recovered by immunopurification of proteasomes with an antisera directed to the PSMA1 subunit (IP 26S) from lysates of mouse fibroblasts exposed to arsenite (25 μM); the content of the lysate before immunopurification was similarly evaluated (input). The asterisk marks nonspecific immunoreactive proteins in the IPs. (C) Immunoblot of endogenous AIRAP, AIRAPL, and the 20S marker PSMA1 in fractions of glycerol gradients prepared from untreated mouse fibroblasts and cells exposed to arsenite (ARS) for 4 h. The migration of complexes of known molecular weights (Mw.) on a parallel gradient is indicated. (D) Immunoblot of endogenous AIRAPL and PSMA1 in proteasomes immunopurified from cells that had been exposed to arsenite without or with cycloheximide (CHX) (to block new protein synthesis). The irrelevant 9E10 antibody controls for specificity. (E) Immunoblot of endogenous PSMA1 and the indicated GST fusion protein recovered in complex by glutathione affinity chromatography from lysates of transfected untreated or arsenite-exposed 293T cells expressing GST (a negative control), AIRAP–GST, AIRAPL–GST, and a chimera consisting of the N terminus of AIRAP fused to the C terminus of AIRAPL at amino acid 140 (AAL.140–GST). (F) Digestion of a small, unstructured peptide [N-succinyl-LLVY-AMC (7-amino-4-methylcoumarin), monitored by fluorescence] in samples of proteasomes purified by glutathione affinity chromatography from 293T cells transfected with AIRAP–GST (AIRAP), AIRAPL–GST (AIRAPL), and a chimera fusing the N-terminal 140 residues of AIRAPL to the C terminus of AIRAP (ALA). PSMD14–GST (PSMD14), a subunit of the 19S proteasome cap served as a reference for the activity of conventional proteasomes and GST as a background control. Where indicated, the inhibitor MG132 (10 μM) was added to the sample before incubation with substrate. (Lower) Arbitrary fluorescence units were normalized to proteasome content assessed by immunoblot for PSMA1.
Despite its similarity to AIRAP, the constitutively expressed AIRAPL was undetectable in proteasomes immunopurified from unstressed cells but was incorporated into proteasomes within 30 min of exposing cells to sodium arsenite (Fig. 3B). AIRAP associated with proteasomes several hours later, after its transcriptional induction. Glycerol gradient centrifugation showed that all of the detectable AIRAPL in lysates of unstressed cells was associated with low molecular weight fractions. Exposure to arsenite shifted some of the AIRAPL to heavier fractions that also contain the AIRAP peak. The arsenic-induced peak of AIRAPL (and AIRAP) colocalized with the heaviest proteasome subunit-containing fractions on the gradient (Fig. 3C), as noted previously in AIRAP's case (11).
The basis for arsenite-induced proteasome association is different for AIRAP and AIRAPL; AIRAP is regulated transcriptionally and, once expressed, associates with proteasomes constitutively (11), whereas AIRAPL is constitutively expressed, and its association with proteasomes is regulated posttranslationally (Fig. 3 D and E). AIRAP and AIRAPL are 60% identical in their proteasome-binding N-terminal domain (amino acids 1–140), but they diverge C-terminal to that (Fig. 3A). The fusion of AIRAPL's C terminus to AIRAP's N-terminal proteasome association domain imparts arsenite regulation to proteasome complex formation (Fig. 3E, lanes 6 and 7), suggesting that AIRAPL's C terminus is able to regulate otherwise constitutive proteasome binding by the N terminus.
The functional consequences of AIRAPL association with proteasomes were compared with those of AIRAP. AIRAP binding to proteasomes enhanced their ability to digest a model small-peptide substrate (11); AIRAPL binding similarly increases peptide hydrolysis (Fig. 3F). This enhancement is most conspicuous when the regulatory C terminus of AIRAPL was replaced by that of the constitutive AIRAP (Fig. 3F, ALA construct). The above experiments indicate that both AIRAP's and AIRAPL's association with proteasomes is regulated (transcriptionally and posttranscriptionally, respectively) and that binding either factor enhances access of a model peptide to the catalytic chamber.
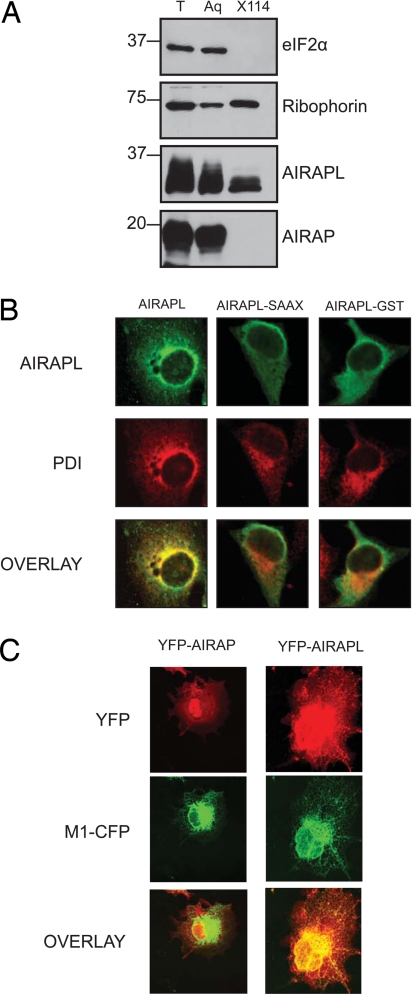
Mammalian AIRAPL and worm AIP-1 have a conserved, functional, ubiquitin-interacting motif(s) (UIMs, Fig. 3A and Fig. S1), and both proteins terminate with a conserved CAAX motif, which serves as an attachment site for a hydrophobic prenyl group. Consistent with this prediction, AIRAPL (but not AIRAP) partitioned into the lipid phase after extraction with Triton X-114 (Fig. 4A). Immunostaining of AIRAPL in transfected COS1 cells revealed an endoplasmic reticulum (ER) pattern, which was abolished by a point mutation, C254S, that inactivates the CAAX box (by converting it to a SAAX) and by a CAAX-disrupting fusion of GST to AIRAPL's C terminus (Fig. 4B). Imaging of live cells expressing a YFP–AIRAPL fusion also revealed an ER pattern overlapping that of the ER marker M1–CFP (21). By contrast, YFP–AIRAP was distributed diffusely throughout the cell (Fig. 4C) (as previously described in ref. 12).
Fig. 4.
AIRAPL associates with ER membranes. (A) 293T cells transiently expressing AIRAP or AIRAPL were lysed by using Triton X-114 and fractionated into aqueous phase (Aq) and detergent phase (X114). Total input (T) and fractions were blotted for AIRAP, AIRAPL, eIF2α (serving as a reference soluble protein), and ribophorin I (serving as a reference membrane-associated protein). (B) COS1 cells transiently expressing the indicated proteins were fixed and immunostained for AIRAPL and PDI (serving as an ER marker). (C) Fluorescence images of live COS1 cells, expressing YFP–AIRAP or YFP–AIRAPL C terminus together with M1–CFP (an ER marker).
AIRAPL is a substrate of the arsenite-activated p38 MAP kinase, which phosphorylates three serine residues in AIRAPL's regulatory C-terminal domain. However, mutation of all three residues to the nonphosphorylatable alanine or the phosphomimetic glutamic acid did not measurably affect arsenite-mediated regulation of proteasome association (Fig. S2). Site-directed mutagenesis also revealed that neither UIM-mediated polyubiquitin binding nor CAAX-dependent membrane binding are required for arsenite-regulated proteasome association (Fig. S3).
Features Common to aip-1 and AIRAPL (but Missing from AIRAP) Support Worm Lifespan.
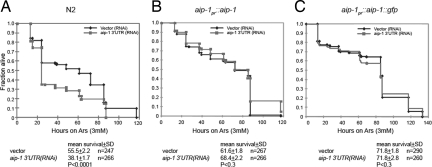
To gain insight into the potential significance of the differences between the proteins encoded by the constitutive AIRAPL gene and the arsenite-inducible AIRAP, we established an experimental system for functional analysis of the protein in aip-1(RNAi) worms. The aip-1(RNAi) phenotype can be recapitulated by targeting the 3′-UTR of the gene because wild-type animals raised on Escherichia coli expressing double-stranded RNA corresponding to aip-1's mRNA 3′-UTR were hypersensitive to arsenite (Fig. 5A). To rescue this phenotype, we experimented with two different transgenes that contain the aip-1 protein coding sequence but lack the 3′-UTR (and are therefore resistant to 3′-UTR-based RNAi, Fig. S4). The hypersensitivity phenotype was rescued both by aip-1pr::aip-1, which encodes a wild-type protein, and by aip-1pr::aip-1::gfp, whose CAAX motif was purposefully corrupted by C-terminal GFP fusion (Figs. 4B and 5 B and C). The latter has the effect of converting AIP-1 from a protein that resembles mammalian AIRAPL to one that resembles AIRAP (which does not associate with membranes, Fig. 4C) and provided an opportunity to compare the two proteins' ability to rescue the effects of aip-1(RNAi) on lifespan.
Fig. 5.
Rescue of the arsenite hypersensitivity phenotype of aip-1(RNAi) by AIP-1-encoding transgenes. (A) Survival plots of arsenite-treated wild-type (N2) animals subjected to aip-1(RNAi) directed to the 3′-UTR of the mRNA or control vector(RNAi). The plots shown are of a typical experiment repeated three times, and cumulative statistics on all three experiments are presented below. (B) As in A except that the animals contain an integrated aip-1pr::aip-1 transgene encoding wild-type AIP-1. (C) As in A except that the animals contain an integrated aip-1pr::aip-1::gfp transgene encoding wild-type AIP-1 fused at its C terminus to GFP, a manipulation that disrupts the CAAX box at the C terminus of the wild-type protein.
Like the coding sequence-directed RNAi (Fig. 1B), targeting aip-1 by its 3′-UTR also reduced lifespan under normal laboratory conditions (Fig. 6A). Interestingly, lifespan shortening by aip-1(RNAi) was rescued by the aip-1pr::aip-1 transgene but not by aip-1pr::aip-1::gfp transgene (Fig. 6 B and C). As expected, neither transgene rescued the effects of daf-16(RNAi) on lifespan. These experiments validate aip-1 as the biologically relevant target of aip-1(RNAi)'s lifespan-shortening effects and suggest that membrane association, which is unique to AIRAPL and missing from AIRAP, is required for normal lifespan.
Fig. 6.
Wild-type AIP-1, but not a C-terminally modified AIP-1, promotes longevity. (A) Survival plots of (N2) animals subjected to aip-1(RNAi) directed to the 3′-UTR, vector(RNAi), and daf-16(RNAi). (B) As in A except that the animals contain an integrated aip-1pr::aip-1 transgene encoding wild-type AIP-1. (C) As in A except that the animals contain an integrated aip-1pr::aip-1::gfp transgene encoding wild-type AIP-1 fused at its C terminus to GFP.
Discussion
Exposure to arsenic threatens protein folding homeostasis and promotes an adaptive transcriptional response that includes cytosolic chaperones (22, 23), proteasome subunits (24), and AIRAP (12). AIRAP's ability to promote substrate access to the proteasome is consistent with an adaptive loosening of proteasomal quality control to facilitate misfolded protein degradation and possibly clearance of trans-inhibitory proteasome substrates in cells exposed to arsenic (11). The analysis of the aip-1 loss-of-function phenotype presented here also implicates AIRAP family proteins in resistance to proteotoxicity under basal conditions and in coping with expression of misfolding-prone proteins that are unrelated to arsenic exposure.
Mammals have two genes encoding AIRAP proteins. The arsenic-inducible ZFAND2a encodes AIRAP (11) and ZFAND2b encoding constitutively expressed AIRAPL. Both proteins share the ability to promote substrate accessibility to the proteasome's catalytic site(s), but AIRAPL has several additional features missing in AIRAP; these include a C-terminal CAAX motif that imparts membrane association and targets the protein to the ER, UIMs that recruit polyubiquitinated proteins to AIRAPL, and a capacity for regulated proteasomal association that requires yet-to-be characterized features of the protein's C terminus.
Caenorhabditis elegans aip-1 encodes a protein with the structural features of AIRAPL and a measure of basal expression (12) that is further induced by arsenite. The worm gene therefore incorporates features of both AIRAP and AIRAPL, and this situation is mirrored in most other animal species; the splitting of the ancestral aip-1 gene into an arsenic-inducible AIRAP and a constitutively expressed AIRAPL is a relatively late feature of evolution apparently restricted to mammals. The inability of C-terminally modified AIP-1::GFP to rescue the lifespan of knockdown animals (Fig. 6) suggests a role for the CAAX box in supporting lifespan. It is tempting to speculate that AIRAPL, which is associated with ER membranes, alters proteasome function in the context of degradation of misfolded secreted or membrane proteins. Our failure, to date, to implicate AIRAPL in ER-associated degradation of misfolded proteins may simply reflect the wrong choice of substrates.
The multidomain AIP-1 and AIRAPL resemble conventional proteasome adaptors. Proteins such as Rad23 and Dsk2 bind the proteasome's 19S cap with one domain [a ubiquitin-like (UBL) domain in the case of RAD23 and the N-terminal AN1 zinc finger in the case of AIRAPL] and recruit polyubiquitinated substrates with other domains [UIM in the case of AIP-1/AIRAPL and a ubiquitin-associated (UBA) domain in the case of RAD23] (25). Compared with these conventional adaptors, AIRAPL has the additional distinction of increasing substrate accessibility at the proteasome, which likely loosens the normally stringent quality control the proteasome exercises over protein degradation (26). The regulated interaction of AIRAPL with proteasomes could restrict AIRAPL activity to conditions in which the waste associated with relaxed proteasome stringency is offset by the benefit of enhanced degradation of potential proteotoxins. AIRAP, which stably interacts with proteasomes and presumably relaxes stringency even further, is transcriptionally induced only under severe stress, as part of a longer-term adaptation. According to this model, the evolution of AIP-1 into AIRAPL and AIRAP affords (long-lived) mammals versatility in their response to the threat of proteotoxicity.
Materials and Methods
Nematode Strains and Growth Conditions.
Standard nematode growth medium was used for C. elegans growth and maintenance. The hsp-16pr::gfp strain has been described previously (11), and the daf-16(mu86), sod-3pr::gfp (CF1553), unc-54pr::Q0–YFP (AM134), unc-54pr::Q35–YFP (AM140), and unc-54pr::Aβ1–42 (CL2006) strains were obtained from the Caenorhabditis Genetics Center. To create the aip-1pr::aip-1::gfp transgene, a 4.4-kb fragment containing the promoter and coding region of aip-1 (with the stop codon removed) was amplified by PCR and ligated into pPD95.75. The aip-1pr::aip-1 construct was then derived from aip-1pr::aip-1::gfp by excising the gfp sequence and reintroducing the aip-1 stop codon. Transgenic worms carrying the various constructs were obtained as described previously (12).
RNAi Feeding, Arsenite Treatment, and Lifespan Studies.
RNAi feeding protocols, bacteria containing aip-1, daf-16, and hsf-1 RNAi vectors, and procedures used to expose worms to arsenite were described previously (12, 27). The 250 bp after the stop codon of aip-1 was amplified by PCR to construct an RNAi feeding vector targeting aip-1's mRNA 3′-UTR. Lifespan assays were conducted at 20–22°C and were initiated at the L4 larvae stage. Animals were transferred away from progeny to new plates until the end of the reproductive period. At least 100 worms were used in each experiment, which were repeated three times. Animals were considered dead when no movement or pharyngeal pumping was observed. XL Stat was used for statistical analysis, and P values were calculated by using the log-rank (Mantel–Cox) method.
Polyglutamine Aggregation and Paralysis Assays.
Polyglutamine reporter animals were grown as described (16). Worm lysates were adjusted to 1% SDS and then layered on a 20% glycerol cushion. Samples were centrifuged at 100,000 × g for 45 min at 4°C. The resulting pellet was resuspended in urea loading buffer and separated by SDS/PAGE for immunoblot. unc-54pr::Aβ1–42 transgenic animals (18) were monitored for paralysis every 24 h.
Mammalian Cell Transfection, Lysis, Staining, and Protein Purification.
A plasmid expressing a GST fusion protein of AIRAPL was constructed by amplifying the coding sequence of the mouse cDNA in-frame with GST in a mammalian expression plasmid. The AIRAP–GST, PSMD14–GST, and M1–CFP mammalian expression vectors were described previously (11). AIRAP (amino acids 1–140) fused to AIRAPL C terminus (amino acids 141–257), AIRAPL (1–257)-GST, and YFP–AIRAP and YFP-AIRAPL (135–257) were constructed by PCR, as was the Cys254→Ser (CAAX→SAAX) mutation. Transfected cells were fixed with ice-cold methanol and stained for AIRAPL (1:250) and protein disulfide isomerase (PDI) as an ER marker (1:100).
Proteasome purification and analysis of enzymatic activity of proteasomes immobilized on glutathione Sepharose beads and normalized for their 20S content by anti-PSMA1 immunoblot was described previously (11).
Antibodies.
Antisera to 9E10, AIRAP, eIF2α, PSMA1, PDI (SPA-891; Stressgen) and ribophorin have been described previously (11). Antisera to bacterially expressed mouse AIRAPL was raised in rabbit and used at dilution of 1:3,000 in immunoblots.
Supplementary Material
Acknowledgments.
We thank Ray Dashaies (California Institute of Technology, Pasadena, CA), David Engelberg (Hebrew University, Jerusalem), Chris Link (University of Colorado, Boulder, CO), Richard Morimoto (Northwestern University, Evanston, IL), and Keiiji Tanaka (Tokyo Metropolitan, Tokyo) for advice and reagents and the Caenorhabditis Genetics Center for mutant worm strains. This work was supported by National Institutes of Health Grants ES08681 and DK47119 (to D.R.), RR017990 and NS050276 (to T.A.N), and F32-DK61179 (to C.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707025105/DCSupplemental.
References
- 1.Soti C, Csermely P. Aging cellular networks: Chaperones as major participants. Exp Gerontol. 2007;42:113–119. doi: 10.1016/j.exger.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Hinault MP, Ben-Zvi A, Goloubinoff P. Chaperones and proteases: Cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J Mol Neurosci. 2006;30:249–265. doi: 10.1385/JMN:30:3:249. [DOI] [PubMed] [Google Scholar]
- 3.Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin Cell Dev Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 5.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama K, et al. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- 7.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Gao B, Lee SM, Bennett K, Fang D. RLE-1, an E3 ubiquitin ligase, regulates C. elegans aging by catalyzing DAF-16 polyubiquitination. Dev Cell. 2007;12:235–246. doi: 10.1016/j.devcel.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Ghazi A, Henis-Korenblit S, Kenyon C. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc Natl Acad Sci USA. 2007;104:5947–5952. doi: 10.1073/pnas.0700638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanhill A, et al. An arsenite-inducible regulatory particle-associated protein (AIRAP) adapts proteasomes to proteotoxicity. Mol Cell. 2006;23:875–995. doi: 10.1016/j.molcel.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Sok J, et al. Arsenite-inducible RNA-associated protein (AIRAP) protects cells from arsenite toxicity. Cell Stress Chaperones. 2001;6:6–15. doi: 10.1379/1466-1268(2001)006<0006:airapa>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 15.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nollen EA, et al. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc Natl Acad Sci USA. 2004;101:6403–6408. doi: 10.1073/pnas.0307697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 18.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 20.Fonte V, et al. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci USA. 2002;99:9439–9444. doi: 10.1073/pnas.152313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu VK, et al. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4:343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 22.Johnston D, Oppermann H, Jackson J, Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980;255:6975–6980. [PubMed] [Google Scholar]
- 23.Levinson W, Oppermann H, Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606:170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- 24.Zheng PZ, et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci USA. 2005;102:7653–7658. doi: 10.1073/pnas.0502825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao H, Sastry A. Recognition of specific ubiquitin conjugates is important for the proteolytic functions of the ubiquitin-associated domain proteins Dsk2 and Rad23. J Biol Chem. 2002;277:11691–11695. doi: 10.1074/jbc.M200245200. [DOI] [PubMed] [Google Scholar]
- 26.Pickart CM, Cohen RE. Proteasomes and their kin: Proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 27.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.