Abstract
Brown algae of the Laminariales (kelps) are the strongest accumulators of iodine among living organisms. They represent a major pump in the global biogeochemical cycle of iodine and, in particular, the major source of iodocarbons in the coastal atmosphere. Nevertheless, the chemical state and biological significance of accumulated iodine have remained unknown to this date. Using x-ray absorption spectroscopy, we show that the accumulated form is iodide, which readily scavenges a variety of reactive oxygen species (ROS). We propose here that its biological role is that of an inorganic antioxidant, the first to be described in a living system. Upon oxidative stress, iodide is effluxed. On the thallus surface and in the apoplast, iodide detoxifies both aqueous oxidants and ozone, the latter resulting in the release of high levels of molecular iodine and the consequent formation of hygroscopic iodine oxides leading to particles, which are precursors to cloud condensation nuclei. In a complementary set of experiments using a heterologous system, iodide was found to effectively scavenge ROS in human blood cells.
Keywords: algae, Laminaria, x-ray absorption spectroscopy, cathodic stripping square wave voltammetry
The element iodine was first isolated from kelp ashes two centuries ago by Courtois in the context of the search for raw materials for explosives (chiefly potassium nitrate) during the Napoleonic Wars (1, 2). Brown algal kelp species such as Laminaria digitata are the most effective known living iodine accumulators, with tissue concentrations often exceeding 50 mM (3). Until the large-scale exploitation of brine waters accompanying natural gas deposits for the recovery of iodine salts (4, 5), Laminariales remained a major commercial source of iodine (6). The importance of iodine for thyroid function is paramount (7). Long before the discovery of this element, the beneficial effect of iodine-rich seaweeds in treating goiter had been recognized initially in China and later in medieval Europe (2). In coastal regions, Laminariales are a major contributor to the iodine flux from the ocean to the atmosphere through the production of volatile halocarbons (8). In addition to iodocarbons, iodine oxide (IO) is also detectable in the atmosphere above kelp beds (9), providing precursors for cloud condensation nuclei (10). Recently, microimaging has revealed that iodine is essentially stored in the extracellular matrix of L. digitata cells located in the peripheral tissues (11). Despite considerable research interest in the role of kelp in the biogeochemical iodine cycle, the identity, speciation, and biological significance of bioaccumulated iodine has remained enigmatic for two centuries. In particular, all studies so far have left significant ambiguity as to the chemical speciation of iodine in Laminaria (6, 11–20).
In this study, we first address the unresolved question of the chemical state of iodine in Laminaria under different physiological conditions, using x-ray absorption spectroscopy (XAS) as a noninvasive probe, and show that the only detectable form of iodine is iodide ion. This is not hydrated as in an aqueous solution of sodium iodide but instead has its hydration shell disrupted by interactions with organic molecules with which it is associated. Upon oxidative stress, high levels of iodide are released and are detectable in the surrounding seawater by voltammetric methods. Using an array of gas chromatography–mass spectrometry (GC-MS), aerosol particle counting, and spectrophotometry to assess both organic and inorganic iodine emissions into the gas phase, we demonstrate that on the thallus surface and in the apoplast, iodide detoxifies both aqueous oxidants and ozone, the latter resulting in the release of molecular iodine and the concomitant, consequent formation of particles, which can act as cloud condensation nuclei. We propose that iodide in kelp constitutes an extracellular protection against oxidative stress and the first identified inorganic antioxidant in a living system.
Results and Discussion
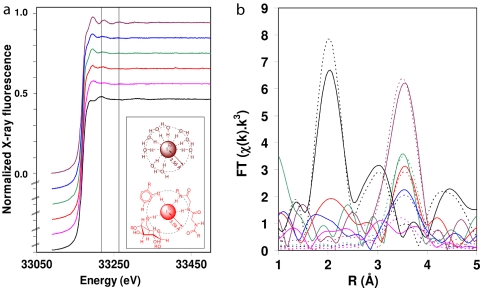
The known reactivity of many iodine species and a hypothesis from the biomedical field (7) led us to investigate its chemical identity in kelp with a noninvasive technique, x-ray absorption spectroscopy (XAS) (21). The iodine K-edge XAS of freshly frozen L. digitata thalli showed a featureless edge (Fig. 1a) and weak EXAFS oscillations [supporting information (SI) Fig. S1]. The corresponding Fourier transform (FT) (Fig. 1b) exhibited no peaks below 3 Å, indicating the absence of covalent bonds to iodine, which is supported by L3-edge measurements (SI Text and Fig. S2). We conclude that iodine is accumulated in Laminaria in its reduced form, iodide. Moreover, the strongest FT peak is at the same distance, ≈3.5 Å, as for hydrated iodide in NaI solution (22), where this peak represents oxygen atoms of the solvent, water. The reduced amplitude in the kelp FT compared with NaI solution is reminiscent of that found upon changing the solvation from small molecules (water) to larger ones (t-butanol, dimethyl formamide) (23). This corresponds to a reduction in the number of surrounding atoms for steric reasons (see Fig. 1a Inset) and an increase in their disorder, indicating that the iodide ion in Laminaria is not surrounded by a complete shell of water oxygens but instead is noncovalently associated with biomolecules such as carbohydrates, polyphenols, or proteins. In line with the desolvation interpretation, lyophilized tissues show only very weak EXAFS (see Fig. 1 for FT; see SI Text and Fig. S1 for EXAFS).
Fig. 1.
XAS of L. digitata tissues, including time course of experiment with fresh Laminaria thalli stressed with oligoguluronates (GG). (a) Iodine XAS (K-edge region). (b) Phase-corrected FT of k3-weighted EXAFS (cf. Fig. S1). Solid lines, experimental; dashed lines, simulations. (Inset) Schematic representation of a cross-section of the immediate environment of the I− ion, showing the change in the solvation by many hydrogen-bonded H2O molecules in 20 mM NaI solution (Upper) to association by fewer hydrogen bonds to biomolecules in fresh Laminaria (Lower; examples clockwise from top left are polyphenol, peptide, and carbohydrate). In Laminaria tissues, iodine is overwhelmingly stored as iodide. In fresh, live tissues, iodide is largely present in association with biomolecules including polyols (e.g., carbohydrates), phenols (e.g., phlorotannins), and amides (e.g., proteins), replacing the highly ordered hydration shell. Upon oxidative stress, part of this iodide is mobilized, as reflected in a more ordered hydration shell. When freeze-dried tissues are exposed to 2 mM hydrogen peroxide in seawater (simulating local concentrations observed during an oxidative burst), much of the available iodine is incorporated into aromatic organic molecules as reflected by the strong change in the EXAFS spectrum (highlighted by the vertical lines at 33,219 eV and 33,266 eV, respectively). Black, lyophilized Laminaria rehydrated in seawater with 2 mM H2O2 [simulation: 1.0 phenyl at 2.1 Å, a = 0.003 Å2 (a, Debye–Waller-type factor as 2σ2 in Å2)]; pink, lyophilized Laminaria (2.2 O at 3.51 Å, a = 0.038 Å2); red, fresh Laminaria (1.6 O at 3.58 Å, a = 0.005 Å2); sea green, 20 min after exposure to GG (2.8 O at 3.58 Å, a = 0.021 Å2); blue, 3 h after exposure to GG (3.9 O at 3.56 Å, a = 0.038 Å2); plum, 20 mM NaI (10.0 O at 3.56 Å, a = 0.034 Å2). A complete list of all simulation parameters with fitting errors is available as Table S1.
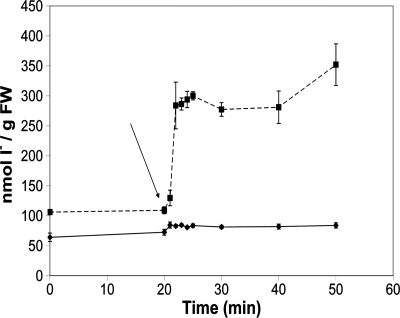
The finding of iodide as the accumulated species and its known reactivity with oxidants (SI Text) prompts questions about its potential role in the context of oxidative stress. We next monitored iodine fluxes in oxidative stress conditions by treating Laminaria thalli with oligoguluronates (GG) (Fig. 2 and Fig. S3). These alginate oligosaccharides are formed by bacterial biofilms and trigger a defense response in Laminaria, involving an oxidative burst (the production of active oxygen species at the cell surface) and potassium efflux (24–26). Using cathodic stripping square wave voltammetry (27), we found a strong release of iodide into the surrounding medium, starting within seconds after GG addition. As shown before under the same experimental conditions, H2O2 concentrations peak at 30 min after elicitation and then decline from a maximum exceeding 10 μM in a typical experimental setup (25). In parallel, up to 2.7 × 10−7 mol I−·g fresh weight−1 (FW) are released within 30 min (Fig. 2, Results and Discussion in SI Text, and Table S2), implying that in a maximum-strength oxidative burst, a Laminaria thallus may lose 0.5% of its accumulated iodide (3). However, iodate and organic iodine levels do not change (data not shown).
Fig. 2.
Time course of iodide efflux during an oligoguluronate-triggered oxidative burst (control, diamonds; GG treatment, squares). The arrow highlights the addition of 100 μg·ml−1 GG, triggering an oxidative burst. At the indicated times, external medium aliquots were taken, and iodide was determined voltammetrically. The data are representative of five experiments.
The XAS edge position is sensitive to the iodine oxidation state (22). The XAS edge (Fig. 1a) of Laminaria tissues did not change noticeably after GG treatment, indicating that the iodine efflux was not accompanied by a change in the oxidation state of the remaining iodine in the alga. Interestingly, the amplitude of the major peak in the FT (Fig. 1b) increased markedly within 20 min after the addition of GG, although it did not reach the level of hydrated iodide in a 20 mM aqueous I− solution. Subsequently, within 3 h after stimulation, it decreased below the level of fresh Laminaria. These changes, also reflected in the occupancies and Debye–Waller-type factors found in the EXAFS simulations (Fig. 1), point to a transition of a significant proportion of the iodide from its biomolecule-associated state in unstressed Laminaria to one more resembling hydrated iodide after 20 min, whereas of the iodide left behind after 3 h, an even larger fraction is biopolymer-associated than is observed in fresh Laminaria. Thus, the release of a small fraction of the free hydrated iodide into the surrounding seawater is only a transient process, and at the end of a stress period, any remaining, hydrated iodide is again sequestrated within the cell wall matrix where it is noncovalently associated with biomolecules. These apparently small but significant changes contrast with the strong change observed in the EXAFS when lyophilized tissues are rehydrated with 2 mM H2O2, namely the appearance of a strong peak at low (2 Å) R in the Fourier transform (Fig. 1 and SI Text). The change is due to the formation of a covalent bond between iodine and a carbon in an aromatic environment (polyphenols, proteins). This result also demonstrates that cell integrity and compartmentation, which are disrupted by lyophilization, are essential in controlling iodine speciation.
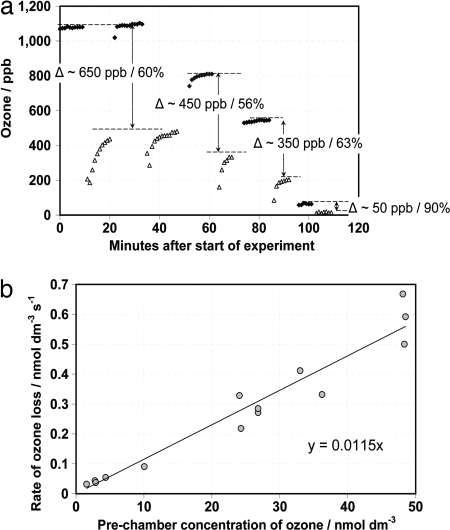
The GG treatment shows that Laminaria has a high capacity to release iodide when subjected to oxidative stress. Because oxidative stress also accompanies the partial emersion of the kelp forest at low tide—e.g., by exposure to high irradiance, desiccation, and atmospheric O3—we simulated low tide in a flow chamber with controlled ozone concentrations. Our experiments (Fig. 3a) revealed the sustained removal of much higher ozone levels than are naturally encountered by Laminaria. Kinetically, this removal process appeared to be first order in [O3] and not limited by the availability of I− even above atmospheric O3 levels. The O3 removal rate (Fig. 3b) was the same in sunlight and in the dark, suggesting a light-independent reaction of aqueous O3 with iodide. O3 reacts extremely rapidly with hydrated I−, forming I2 (28) (SI Text). The rate constant for ozone removal (0.0115 s−1; Fig. 3b) along with typical values of aerodynamic resistance yields a deposition velocity due only to liquid phase diffusion and chemical reactions (1/rl) in the range 0.25–1 cm·s−1 (SI Text). Thus, emerged kelp beds scavenge ozone at least an order of magnitude faster than seawater (≈0.03 cm·s−1) (29), impacting atmospheric chemistry.
Fig. 3.
Scavenging of ozone by Laminaria. (a) Concentrations of O3 in a 2 liter·min−1 flow of zero air (i.e., particle-free, scrubbed) measured before (diamonds) and after (triangles) a glass chamber containing ≈5 g FW (surface area ≈100 cm2) Laminaria. A range of different O3 concentrations were applied over the course of one experiment, starting with high concentrations that were reduced in steps. Switching the O3 measurement flow from before to after the chamber or vice versa necessitated temporarily disturbing the total flow, hence the several minutes taken for the O3 levels to stabilize. Measurements made before the chamber are shown as filled symbols, and those made after the chamber are shown as open symbols. Experiments with an empty chamber show <8% steady-state loss of O3 to chamber walls. (b) Rate of ozone loss over ≈100-cm2 strips of L. digitata in the flow chamber. The data are representative of 14 independent experiments. For comparison, typical O3 concentrations in the marine boundary layer are of the order of a few tens of parts per billion.
It is well established that aerosol particle bursts occur in the air over kelp fields at low tide during daytime (10). We have previously observed that particles (>3 nm) were formed only under conditions such that Laminaria was in direct contact with ozone and in the light (30), when we also measured I2 fluxes from the seaweed surface at rates as high as 130 pmol·min−1·g FW−1 (averaged over a 1-h period and at 160 ppbv O3). These results suggest that particles are formed as a consequence of the photolysis of I2 formed on the seaweed surface by the abiotic reaction O3 + I− (30). This is consistent with recent reports (31) that I2 is the major source of I atoms in the air over seaweed beds. In contrast to previous studies attributing the rapid appearance of high concentrations of recently formed ultrafine aerosol particles (most commonly referred to as coastal particle bursts) to iodocarbon emissions (10), our study suggests that they are rather a consequence of the ozone-scavenging reactivity of iodide on kelp thallus surfaces. Freeze-dried Laminaria exposed to O3 produced even higher quantities of aerosol particles than fresh seaweed, suggesting that the particle response is due to a greater reactivity with the iodide reservoir of dead Laminaria tissue (lacking cell compartmentation). Unlike in living thalli, the particle response did not recover with freeze-dried samples after the ozone exposure was temporarily halted. This further reinforces the finding that biotic processes are important in the iodine accumulation and release, but not in particle formation.
Iodide is an effective antioxidant in heterologous systems that naturally do not accumulate iodide—e.g., blocking the oxidative burst in human neutrophils (IC50 = 2.9 mM; Fig. S4). Growth experiments of marine bacteria and the analysis of toxicity to human leukocytes by measuring the mitochondrial membrane potential with flow cytometry revealed that iodide is nontoxic to both pro- and eukaryotic cells (SI Text and Fig. S5). A direct defense function of high iodide concentrations can therefore be excluded; however, under apoplastic oxidative stress conditions and as a substrate of haloperoxidases, iodide can undergo a range of reactions leading to toxic products. Because the XAS data show that the accumulated iodine remains in the reduced state, this would affect only a minute fraction of the total iodine. This may nevertheless be sufficient for an additional defense function considering, e.g., the high toxicity of iodocarbons, which are indeed emitted at increased rates after elicitation with GG (30).
Major groups of antioxidants of photosynthetic organisms are organic compounds such as ascorbate, isoprenoids, fatty acids, and phenols (32). We observed ≈10−4 M ascorbate and 10−3 M glutathione in Laminaria. At these concentrations and cellular pH, their reduction potentials are considerably more negative than that of the iodide/iodate couple—i.e., they would be oxidized before iodide if present in the same compartment. Yet the highest H2O2 concentrations (>1 mM) after an oxidative burst are encountered not in the cytosol but in the cell wall space (apoplast) (25), which is in free diffusion exchange with the surrounding seawater. It is this compartment where iodide is accumulated (and mobilized during oxidative stress); yet ascorbate and glutathione are strictly intracellular, with apoplastic excretion being detected neither in this study (data not shown) nor in any other living systems. Interestingly, a recent expressed sequence tag (EST) study in L. digitata protoplasts highlighted only a few of the enzymes scavenging reactive oxygen species (ROS) that are typically strongly expressed by eukaryotes during oxidative stress (33), and L. digitata contains fewer soluble phenols that are considered antioxidants in other brown algae (34). Furthermore, H2O2 excretion appears widespread among macroalgae (26). In the absence of ascorbate and glutathione and with up to millimolar levels of ROS present in the apoplast, iodide is indeed a potent antioxidant. Finally, it should be highlighted that an extracellular antioxidant role of iodide in Laminaria fits well with the observed seasonality of iodine levels in this kelp: A recent field survey of European Laminaria populations showed reductions of iodine content of 50% or more during the summer months, when kelp forests are subject to substantial temperature and photooxidative stress (35).
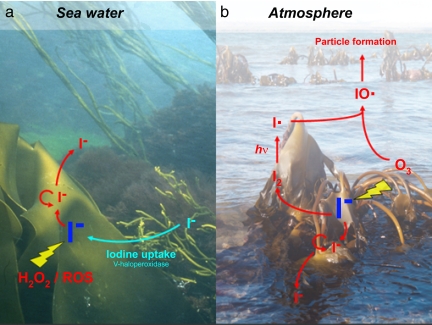
We therefore conclude that iodide accumulation leads to an apoplastic antioxidant reservoir that can be mobilized during oxidative stress (Fig. 4). Iodide is well suited as a versatile antioxidant, scavenging not only aqueous H2O2 and gaseous O3, as demonstrated in this study, but also hydroxyl radicals and superoxide. Based on thermodynamic and kinetic data (36–38), the reaction of iodide with ROS (H2O2, O3, O2−, 1O2, and HO2•; Tables S3 and S4 and Results and Discussion in SI Text) is more favorable than that of chloride or bromide. At the concentrations present at the immediate cell membrane surface, approaching levels of 10–100 mM, iodide reacts with H2O2, catalyzed by high activities of the halo-/iodoperoxidases present (39). Whereas the nonenzymatic reaction of I− with H2O2 is slow, the reactions with O3, O2−, 1O2, and OH• are very fast (38): iodide reacts with three of these four ROS (ozone, singlet oxygen, and superoxide radicals) at rates 12- to 500-fold higher than ascorbate and glutathione. On the basis of these kinetic data, iodide is the best available antioxidant in the Laminaria apoplast. HOI is the central, short-lived intermediate for all oxidants (reactions 9, 10, 12, and 13 in Table S3) except for ozone and singlet oxygen in the gas phase (reactions 8 and 11, respectively, in Table S3). In the absence of organic substrates, haloperoxidases catalyze rapid halide-mediated disproportionation of H2O2 (see Results and Discussion in SI Text). In the presence of organic substrates, iodide incorporated in them would be regenerated rapidly by nucleophilic substitution with Cl−, Br−, or HO− (Table S5). Thus, for most oxidants except ozone, there would be no buildup of oxidized or dissolved organic iodine species, as confirmed by our experiments. Iodocarbons such as diiodomethane and iodoform are emitted by Laminaria at increased rates after an oxidative burst, albeit at rates amounting to <0.1% of the inorganic iodide efflux observed here (8, 30) and a similarly small stoichiometric proportion compared with the H2O2 that is first generated and then degraded in the oxidative burst (25). Therefore, iodocarbons cannot account for the scavenging of ROS. We propose instead a defense function for iodocarbons, considering their high microbial toxicity due to the effectiveness of iodide as a leaving group in nucleophilic substitutions.
Fig. 4.
A model of iodine metabolism in Laminaria. Laminaria, when submerged and unstressed (a), accumulates iodide from seawater mediated by vanadium haloperoxidase (turquoise). In the tissues and as shown in this study, I− is the accumulated form. When oxidative stress occurs (red), iodide is released to detoxify ROS in the apoplast at the thallus surface outside of the cell membrane, including both aqueous (e.g., H2O2) and gaseous (O3) oxidants. ROS scavenging reactions in the aqueous phase such as halide-assisted disproportionation of H2O2 will result in the regeneration of iodide in a cyclic reaction sequence. (a) During oxidative stress at high tide (e.g., due to an oxidative burst caused by alginate-degrading bacteria in a biofilm), iodide is released into the surrounding seawater. (b) In contrast, aerosol particle bursts result mainly from molecular iodine released directly from Laminaria into the coastal atmosphere, as a consequence of the ozone-scavenging reactivity of iodide on kelp surfaces at low tide.
This study significantly deepens our understanding of the contribution of kelp forests to iodine chemistry in coastal atmospheric and marine systems. Their potential to excrete iodide at rates several orders of magnitude higher than organic iodine compounds has not been recognized previously, although kelp is well established as a major source of iodocarbons (8). To our knowledge, this describes the first inorganic and also the chemically simplest antioxidant—a single, negatively charged atom—with a central role for marine and atmospheric processes through the provision of condensation nuclei and the removal of ozone.
Materials and Methods
Biological Material.
Laminaria digitata sporophytes were collected in Helgoland (German Bight) and Roscoff (Brittany, France) and kept alive in aerated, running seawater tanks at 4–6°C. Their approximate iodine content was 1% dry weight (apoplastic concentration ≈20 mM) (3).
Oxidative Stress Experiments.
Oligoguluronate elicitors (GG) were prepared as described in ref. 25 and applied at a final concentration of 100 μg·ml−1. Typically, experiments were conducted with ≈5 g of Laminaria fresh weight in 100 ml of natural seawater, from which both tissue samples of ≈0.5 g and aliquots of 15 ml of seawater medium were removed directly at the onset of the stress, and then at intervals of 1, 3, 5, 10, 15, 30, 45, 60, and 180 min and 24 h. For XAS analysis, L. digitata tissue samples were cut out of the phylloid blade. They were fitted into a Plexiglas frame and sealed with Capton tape. All samples were immediately frozen in liquid nitrogen for subsequent XAS analysis. This experiment was repeated five times with minor variations in sample volume, total algal biomass, and seawater volume.
X-Ray Absorption Spectroscopy.
Iodine K-edge XAS measurements (22) were carried out at the European Molecular Biology Laboratory, Hamburg Unit, Outstation Hamburg at Deutsches Elektronen Synchrotron, Germany, using a Si(311) order sorting monochromator, which was set at 50% of peak intensity to suppress harmonics. During data collection, the storage ring DORIS III was operated at 4.5 GeV with ring currents between 150 and 90 mA. The x-ray absorption spectra were measured in fluorescence mode with a CANBERRA 13 element solid-state detector. Scans at the I K-edge were calibrated by reference to the absorption spectrum of a NaI sample. Typically, 15 scans per sample were taken. The sample was kept at 20 K during the measurements and moved in between scans so that the part of the sample that was exposed to the beam was varied as much as possible. No spectroscopic differences indicative of photoreduction were observed between successive scans. Iodine L3-edge XAS data were collected in fluorescence mode at the Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland, microXAS beamline X05LA. Sample temperature was adjusted to ≈100 K by a nitrogen cryostream. Neither ice formation on the beam spot nor photoreduction in the scans shown here (SI Text) has been observed.
EXAFS Data Reduction and Analysis.
Data reduction was carried out as described in ref. 22. Simulations of the calibrated, averaged, and background-subtracted EXAFS were carried out with the Windows XP version of the EXAFS simulation program EXCURVE (40), Release 9.272, using the Rehr and Albers expansion for the calculation of phase shifts (41).
Supplementary Material
Acknowledgments.
This work was supported by the Natural Environment Research Council (L.J.C.); the SAMS Core Strategic Program (F.C.K.); the Deutsche Forschungsgemeinschaft (P.M.H.K.); the U.S. National Institutes of Health; the Center for Environmental Bioinorganic Chemistry, a U.S. National Science Foundation Environmental Molecular Science Institute (A.B.); the French National Program “Toxicologie Nucléaire Environnementale” (P.P.); the National Science Foundation (G.W.L.); and the National Oceanic and Atmospheric Administration (G.W.L.). Furthermore, the authors are grateful for support from the European Community in the framework of the Access to Research Infrastructure Action of the Improving Human Potential Program to the European Molecular Biology Laboratory Hamburg Outstation. Finally, the staff of the Alfred Wegener Institute/Biologische Anstalt Helgoland are thanked for providing Laminaria digitata.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709959105/DCSupplemental.
References
- 1.Courtois B. Découverte d'une substance nouvelle dans le Vareck. (Discovery of a new substance in kelp.) Ann Chim (Paris) 1813;88:304–310. [Google Scholar]
- 2.Rosenfeld L. Discovery and early uses of iodine. J Chem Educ. 2000;77:984–987. [Google Scholar]
- 3.Küpper FC, et al. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta. 1998;207:163–171. [Google Scholar]
- 4.Amachi S, et al. Isolation of iodide-oxidizing bacteria from iodide-rich natural gas brines and seawaters. Microb Ecol. 2005;49:547–557. doi: 10.1007/s00248-004-0056-0. [DOI] [PubMed] [Google Scholar]
- 5.Lyday PA. USGS Mineral Commodity Summaries. Washington, DC: United States Geol Surv; 1998. pp. 82–83. [Google Scholar]
- 6.Bartsch I, et al. The genus Laminaria sensu lato: Recent insights and developments. Eur J Phycol. 2008;43:1–86. [Google Scholar]
- 7.Venturi S, Venturi M. Iodide, thyroid and stomach carcinogenesis: Evolutionary story of a primitive antioxidant? Eur J Endocrinol. 1999;140:371–372. doi: 10.1530/eje.0.1400371. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter LJ, Malin G, Küpper FC, Liss PS. Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal East Atlantic. Global Biogeochem Cycles. 2000;14:1191–1204. [Google Scholar]
- 9.Alicke B, Hebestreit K, Stutz J, Platt U. Iodine oxide in the marine boundary layer. Nature. 1999;397:572–573. [Google Scholar]
- 10.O'Dowd CD, et al. Marine aerosol formation from biogenic iodine emissions. Nature. 2002;417:632–636. doi: 10.1038/nature00775. [DOI] [PubMed] [Google Scholar]
- 11.Verhaeghe EF, et al. Microchemical imaging of iodine distribution in the brown alga Laminaria digitata suggests a new mechanism for its accumulation. J Biol Inorg Chem. 2008 doi: 10.1007/s00775-007-0319-6. in press. [DOI] [PubMed] [Google Scholar]
- 12.Amat M, Srivastava LM. Translocation of iodine in Laminaria saccharina (Phaeophyta) J Phycol. 1985;21:330–333. [Google Scholar]
- 13.Eschle NN. Ueber den Jodgehalt einiger Algenarten. (On the iodine content of some algae species.) Z Physiol Chem. 1897;23:30–37. [Google Scholar]
- 14.Hou XL, Chai CF, Qian QF, Yan XJ, Fan X. Determination of chemical species of iodine in some seaweeds. Sci Total Environ. 1997;204:215–221. [Google Scholar]
- 15.Kylin H. Über das Vorkommen von Jodiden, Bromiden und Jodidoxydasen bei Meeresalgen. (On the occurence of iodides, bromides, and iodide oxidases in marine algae.) Hoppe-Seyler's Z Physiol Chem. 1929;186:50–84. [Google Scholar]
- 16.Roche J, Yagi Y. Sur la fixation de l'iode radioactif par les algues et sur les constituants iodés des Laminaires. (About the fixation of radioactive iodine by algae and iodinated constituents in kelps.) Compt Rend Soc Biol (Paris) 1952;146:642–645. [PubMed] [Google Scholar]
- 17.Shah M, Wuilloud RG, Kannamkumaratha SS, Caruso JA. Iodine speciation studies in commercially available seaweed by coupling different chromatographic techniques with UV and ICP-MS detection. J Anal Atom Spectrom. 2005;20:176–182. [Google Scholar]
- 18.Shaw T. The mechanism of iodine accumulation by the brown sea weed Laminaria digitata. The uptake of 131I. Proc R Soc London Ser B. 1959;150:356–371. doi: 10.1098/rspb.1959.0027. [DOI] [PubMed] [Google Scholar]
- 19.Shaw TI. The mechanism of iodine accumulation by the brown sea weed Laminaria digitata. Respiration and iodide uptake. Proc R Soc London Ser B. 1960;152:109–117. doi: 10.1098/rspb.1960.0027. [DOI] [PubMed] [Google Scholar]
- 20.Tong W, Chaikoff IL. Metabolism of 131I by the marine alga, Nereocystis luetkeana. J Biol Chem. 1955;215:473–484. [PubMed] [Google Scholar]
- 21.George GN, Hedman B, Hodgson KO. An edge with XAS. Nat Struct Biol Synchrotron. 1998;(Suppl):645–647. doi: 10.1038/1336. [DOI] [PubMed] [Google Scholar]
- 22.Feiters MC, Küpper FC, Meyer-Klaucke W. X-ray absorption spectroscopic studies on model compounds for biological iodine and bromine. J Synchrotron Radiat. 2005;12:85–93. doi: 10.1107/S0909049504027815. [DOI] [PubMed] [Google Scholar]
- 23.Tanida T, Watanabe I. Dependence of EXAFS (Extended X-Ray Absorption Fine Structure) parameters of iodide anions in various solvents upon a solvent parameter. Bull Chem Soc Jpn. 2000;73:2747–2752. [Google Scholar]
- 24.Küpper FC, et al. Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades. J Exp Bot. 2006;57:1991–1999. doi: 10.1093/jxb/erj146. [DOI] [PubMed] [Google Scholar]
- 25.Küpper FC, Kloareg B, Guern J, Potin P. Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiol. 2001;125:278–291. doi: 10.1104/pp.125.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Küpper FC, Müller DG, Peters AF, Kloareg B, Potin P. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of the sporophytes of Laminariales. J Chem Ecol. 2002;28:2057–2081. doi: 10.1023/a:1020706129624. [DOI] [PubMed] [Google Scholar]
- 27.Luther GW, III, Swartz CB, Ullman WJ. Direct determination of iodide in seawater by cathodic stripping square wave voltammetry. Anal Chem. 1988;60:1721–1724. [Google Scholar]
- 28.Liu Q, et al. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Inorg Chem. 2001;40:4436–4442. doi: 10.1021/ic000919j. [DOI] [PubMed] [Google Scholar]
- 29.Chang WN, Heikes BG, Lee MH. Ozone deposition to the sea surface: Chemical enhancement and wind speed dependence. Atmos Environ. 2004;38:1053–1059. [Google Scholar]
- 30.Palmer CJ, Anders TL, Carpenter LJ, Küpper FC, McFiggans GB. Iodine and halocarbon response of Laminaria digitata to oxidative stress and links to atmospheric new particle production. Environ Chem. 2005;2:282–290. [Google Scholar]
- 31.McFiggans GB, et al. Direct evidence for coastal iodine particles from Laminaria macroalgae—linkage to emissions of molecular iodine. Atmos Chem Phys. 2004;4:701–713. [Google Scholar]
- 32.Demmig-Adams B, Adams WWI. Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- 33.Roeder V, et al. Identification of stress gene transcripts in Laminaria digitata (Phaeophyceae) protoplast cultures by expressed sequence tag analysis. J Phycol. 2005;41:1227–1235. [Google Scholar]
- 34.Connan S, Delisle F, Deslandes E, Gall EA. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar. 2006;49:39–46. [Google Scholar]
- 35.ArGall E, Küpper FC, Kloareg B. A survey of iodine contents in Laminaria digitata. Bot Mar. 2004;47:30–37. [Google Scholar]
- 36.Stumm W, Morgan JJ. Aquatic Chemistry. New York: Wiley; 1996. [Google Scholar]
- 37.Stanbury DM. In: Advances in Inorganic Chemistry. Sykes AG, editor. Orlando, FL: Academic; 1989. pp. 167–211. [Google Scholar]
- 38.Luther GW, III, Wu J, Cullen JB. Redox chemistry of iodine in seawater: Frontier molecular orbital theory considerations. Aquatic Chemistry. In: Huang CP, O'Melia CR, Morgan JJ, editors. Interfacial and Interspecies Processes, Advances in Chemistry Series. Vol 244. Washington, DC: Am Chem Soc; 1995. pp. 134–155. [Google Scholar]
- 39.Colin C, et al. The brown algal kelp Laminaria digitata features distinct bromoperoxidase and iodoperoxidase activities. J Biol Chem. 2003;278:23545–23552. doi: 10.1074/jbc.M300247200. [DOI] [PubMed] [Google Scholar]
- 40.Gurman SJ, Binsted N, Ross I. A rapid, exact, curved-wave theory for EXAFS calculations. II. The multiple-scattering contributions. J Phys C Solid State Phys. 1986;19:1845–1861. [Google Scholar]
- 41.Rehr JJ, Albers RC. Scattering matrix formulation of curved-wave multiple-scattering theory: Application to XAFS. Phys Rev B. 1990;41:8139–8149. doi: 10.1103/physrevb.41.8139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.