Abstract
Mitochondria form dynamic tubular networks that undergo frequent morphological changes through fission and fusion, the imbalance of which can affect cell survival in general and impact synaptic transmission and plasticity in neurons in particular. Some core components of the mitochondrial fission/fusion machinery, including the dynamin-like GTPases Drp1, Mitofusin, Opa1, and the Drp1-interacting protein Fis1, have been identified. How the fission and fusion processes are regulated under normal conditions and the extent to which defects in mitochondrial fission/fusion are involved in various disease conditions are poorly understood. Mitochondrial malfunction tends to cause diseases with brain and skeletal muscle manifestations and has been implicated in neurodegenerative diseases such as Parkinson's disease (PD). Whether abnormal mitochondrial fission or fusion plays a role in PD pathogenesis has not been shown. Here, we show that Pink1, a mitochondria-targeted Ser/Thr kinase linked to familial PD, genetically interacts with the mitochondrial fission/fusion machinery and modulates mitochondrial dynamics. Genetic manipulations that promote mitochondrial fission suppress Drosophila Pink1 mutant phenotypes in indirect flight muscle and dopamine neurons, whereas decreased fission has opposite effects. In Drosophila and mammalian cells, overexpression of Pink1 promotes mitochondrial fission, whereas inhibition of Pink1 leads to excessive fusion. Our genetic interaction results suggest that Fis1 may act in-between Pink1 and Drp1 in controlling mitochondrial fission. These results reveal a cell biological role for Pink1 and establish mitochondrial fission/fusion as a paradigm for PD research. Compounds that modulate mitochondrial fission/fusion could have therapeutic value in PD intervention.
Keywords: Drosophila, Parkinson's disease, muscle, neurodegeneration, mitochondrial morphogenesis
Parkinson's disease (PD) is the most common movement disorder characterized by relatively selective degeneration of dopamine (DA) neurons. Postmortem studies have identified common features associated with PD, such as mitochondrial dysfunction, oxidative stress, and aggregation of abnormal proteins (1, 2). Although most PD cases are sporadic, recent studies have highlighted the importance of genetic factors in PD pathogenesis (2, 3). At least 10 distinct loci have been associated with familial forms of PD (FPD). Understanding the molecular lesions associated with these FPD genes promises to shed light on the pathogenesis of the more common forms of disease. Interestingly, studies using animal models and cell culture models have linked mutations of FPD genes to impairments of mitochondrial structure/function. Furthermore, some of these disease-associated proteins have been shown to be present in mitochondria or interact with mitochondrial proteins (4–7), reinforcing the general involvement of mitochondrial dysfunction in PD pathogenesis.
Recent studies of Pink1 have provided direct support for a causal role of mitochondrial dysfunction in PD pathogenesis. Pink1 encodes a predicted Ser/Thr kinase with a predicted mitochondrial targeting signal (5). In Drosophila, inactivation of dPink1 leads to indirect flight muscle (IFM) degeneration, DA neuron loss, photoreceptor loss, and male sterility (8–11). These effects are generally accompanied by morphological and functional defects in the mitochondria of the affected tissues. Significantly, at least in IFM, the occurrence of abnormal mitochondria precedes tissue degeneration (8), supporting a causative role for mitochondrial dysfunction in dPink1-induced muscle cell death. Furthermore, genetic studies in Drosophila have revealed that Parkin acts downstream of Pink1 to maintain mitochondrial function and tissue integrity (8–10).
Despite these studies, the exact mitochondrial process that is regulated by Pink1 remains elusive. Intrigued by the mitochondrial morphological phenotypes induced by dPink1 inactivation, we have examined whether Pink1 regulates mitochondrial fission/fusion, which are dynamic membrane remodeling processes that govern mitochondrial morphology in eukaryotes (12). Our results indicate that dPink1 exhibits genetic interactions with components of the mitochondrial fission/fusion machinery and it regulates mitochondrial dynamics. Our findings link mitochondrial fission/fusion to PD pathogenesis and suggest ways to understand and treat PD and related disorders.
Results
Genetic Interactions Between Pink1 and Components of the Mitochondrial Fission and Fusion Pathways.
Swollen mitochondria appeared in IFM of dPink1 mutant before overt myofiber degeneration (8), suggesting that defective mitochondrial morphogenesis is a primary effect of dPink1 inactivation. To test this possibility, we manipulated the activities of the mitochondrial fission and fusion pathways. Increasing mitochondrial fission by introducing an extra copy of Drp1 (13), a key component of the fission machinery, efficiently rescued the wing posture phenotype caused by IFM degeneration in dPink1 mutant [Fig. 1 B and F and supporting information (SI) Tables S1 and S2]. Conversely, Drp1 heterozygosity significantly enhanced dPink1 mutant phenotype (Table S1). Heterozygosity of Opa1-like (14), a core component of the fusion machinery, also significantly suppressed dPink1 mutant phenotype (Fig. 1 C and G and Tables S1 and S2), although the effect subsided in older animals. Similar manipulation of Drp1 or Opa1-like did not affect a retinal degeneration phenotype caused by inhibition of DJ-1a (15), a Drosophila homologue of another familial PD gene affecting mitochondrial function (Fig. S1). This result demonstrates specificity of the genetic interactions between dPink1 and the mitochondrial fission/fusion genes.
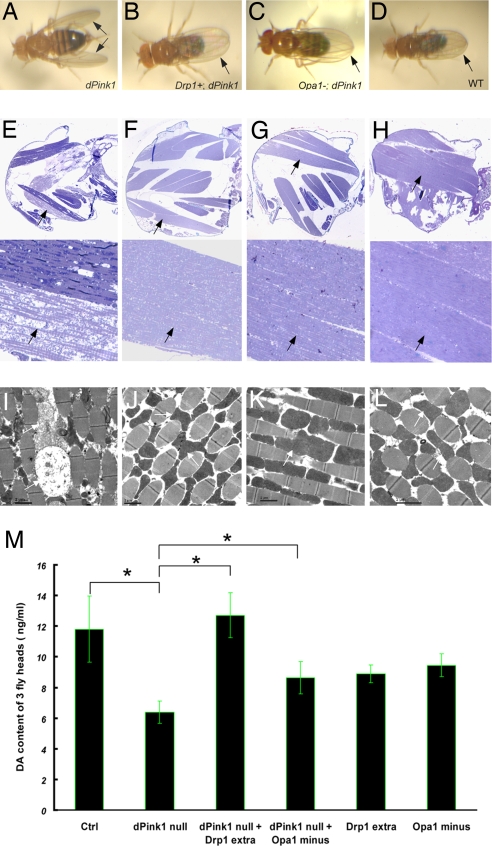
Fig. 1.
Promotion of mitochondrial fission suppresses mutant phenotypes caused by dPink1 loss of function. (A–D) Wing posture phenotypes in 5-day-old adult flies of the following genotypes: dPink1B9 (or B9) (A), dPink1B9; Drp1 extra copy (B), dPink1B9; Opa1-like/+ (C), and wild-type control (D). The arrows point to the abnormal wing posture in A and normal posture in B–D. (E–H) (Upper) Longitudinal sections of thoraces in 5-day-old adult flies of the following genotypes: dPink1B9 (E), dPink1B9; Drp1 extra copy (F), dPink1B9; Opa1-like/+ (G), and control (H). (Lower) Magnified views of corresponding IFMs. Sections from resin-embedded thoraces of 5-day-old adult flies were stained with toluidine blue to visualize tissue morphology, particularly the musculatures; anterior is to the left. The arrows point to IFMs. (I–L) Transmission electronic microscopy analysis of IFM ultrastructure in 5-day-old adult flies of the corresponding genotypes shown above. The arrows point to mitochondria. (M) Added expression of Drp1 or loss of one copy of Opa1-like suppressed the reduction of head dopamine (DA) content caused by loss of dPink1. Two-week-old flies were subject to DA measurement. *, P < 0.01, one-way ANOVA test.
We next examined tissue integrity and mitochondrial morphology in the IFM of rescued animals. In control animals, spindle-shaped IFM fibers were aligned in an organized manner, with mitochondria packed in-between muscle fibers (Fig. 1L). These mitochondria contained electron-dense material and cristae. IFM fibers were thin, atrophic, and disorganized in dPink1 mutant (Fig. 1I). Some of the mitochondria appeared grossly enlarged, with inner membrane disorganized or disintegrated and overall electron density decreased (Fig. 1I). Drp1 overexpression restored mitochondrial morphology and IFM morphology and organization (Fig. 1J). Opa1-like heterozygosity also showed effective rescue (Fig. 1K). Drp1 overexpression or Opa1-like heterozygosity had little effect on IFM organization or mitochondrial morphology in an otherwise wild-type background (Fig. S2). The rescue of IFM pathology of dPink1 mutant by the mitochondrial fission and fusion genes thus correlates with the restoration of mitochondrial morphology.
To test whether the genetic interaction between dPink1 and mitochondrial fission/fusion genes happens in other tissues, we analyzed DA neurons. Inactivation of dPink1 leads to dysfunction of DA neurons, as indicated by the reduction of brain DA levels (8, 9). Drp1 overexpression restored DA levels to normal in dPink1-null mutant background (Fig. 1M). Opa1-like heterozygosity also showed rescuing effect (Fig. 1M), although not as robust as Drp1 overexpression. dPink1 mutants overexpressing Drp1 or heterozygous for Opa1-like have a normal number of dopaminergic neurons (data not shown). It should be noted that the degree of brain DA-level reduction (≈50%) is much greater than the minor or no-DA-neuron loss seen in dPink1-null mutants (9, 10), suggesting that, in addition to promoting DA neuron survival, Pink1 may play a more prominent role in regulating dopaminergic physiology, such as DA metabolism, storage, or transmission.
Pink1 Cooperates with Mitochondrial Fission/Fusion Proteins to Regulate Mitochondrial Morphogenesis in DA Neurons.
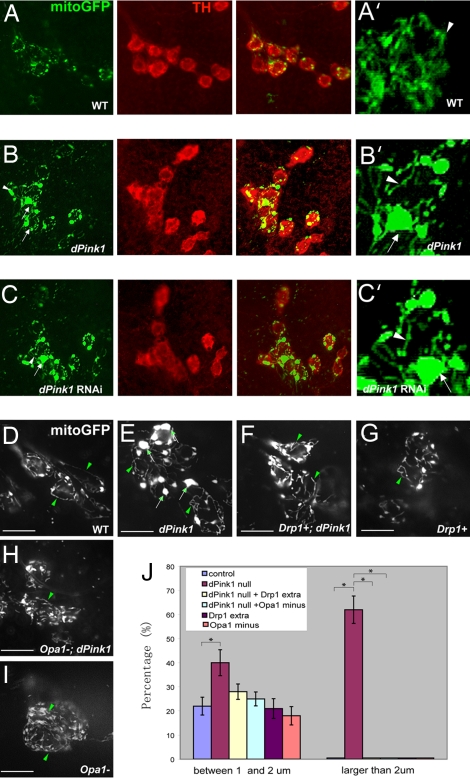
We next examined the effect of manipulating fission/fusion genes on mitochondrial morphology in the DA neurons of dPink1 mutant by using a mito-GFP reporter that targets GFP to the mitochondrial matrix. Mito-GFP was expressed in DA neurons using the tyrosine hydroxylase (TH)-Gal4 driver. The mito-GFP reporter clearly labeled mitochondrial networks, which contained tubular and punctate units (Fig. 2A, A′, and J). We focused our analysis on DA neurons in the dorsolateral protocerebral posterior (PPL1 or DL1) clusters. In dPink1 mutant or dPink1 RNAi animals, prominent mitochondrial aggregates formed. In addition, tubular structures were frequently observed (Fig. 2 B, B′, C, C′, E, and J). Mito-GFP aggregates were also observed in other DA neuron clusters of dPink1 mutant (data not shown). In dPink1 mutant overexpressing Drp1, however, mitochondrial aggregates were no longer formed, and mito-GFP signals were uniformly distributed throughout the soma (Fig. 2 F and J), as seen in the control (Fig. 2 A, A′, D, and J). Removal of one copy of Drp1 reversed the rescuing effect of Drp1 overexpression conferred by the extra copy (Fig. S3A). Drp1 overexpression by introducing an extra copy in an otherwise wild-type background showed no obvious effect on mitochondrial network morphology (Fig. 2 G and J). Similarly, Opa1-like heterozygosity also had no obvious effect in a wild-type background (Fig. 2 I and J), but it suppressed mitochondria aggregation in dPink1 mutant (Fig. 2 H and J). In contrast, loss of one copy of the mitochondrial manganese superoxide dismutase (MnSODII) gene had no effect on dPink1 mutant phenotype (Fig. S3D). These results are consistent with dPink1 specifically affecting mitochondrial fission/fusion dynamics.
Fig. 2.
Up-regulation of mitochondrial fission prevents mitochondrial clustering in DA neurons caused by loss of dPink1. (A–C) Whole-mount immunostaining of dorsolateral protocerebral posterior (PPL1 or DL1) cluster DA neurons in 5- to 7-day-old adult flies of the following genotypes: TH-Gal4>UAS-mitoGFP (A), TH-Gal4>UAS-mitoGFP; dPink1B9 (B), and TH-Gal4>UAS-mitoGFP; UAS-dPink1 RNAi (C). (A–C) (Left) Immunostaining of GFP to monitor mitochondria. (Center) Immunostaining of TH to mark DA neurons. (Right) Merged images. (D–I) Live imaging of mitochondria by visualizing mitoGFP within the PPL1 cluster neurons in 5- to ≈7-day-old adult flies of the following genotypes: TH-Gal4>UAS-mitoGFP (D), TH-Gal4>UAS-mitoGFP; dPink1B9 (E), TH-Gal4>UAS-mitoGFP; dPink1B9; Drp1 extra copy (F), TH-Gal4>UAS-mitoGFP; Drp1 extra copy (G), TH-Gal4>UAS-mitoGFP; dPink1B9, Opa1-like/+ (H), and TH-Gal4>UAS-mitoGFP; Opa1-like/+ (I). A′, B′, and C′ show higher magnification views of the mitochondrial networks in A–C, respectively. Arrows, aggregated mitochondria; arrowheads, tubular mitochondria. (J) Quantification of the percentage of DA neurons containing mitochondrial aggregates with diameters of 1–2 or >2 μm. Note that mitochondrial aggregates >2 μm in diameter were observed almost exclusively in dPink1 mutant. *, P < 0.01, one-way ANOVA test.
Pink1 Overexpression also Leads to Abnormal Mitochondrial Morphogenesis in DA Neurons.
To test whether dPink1 is sufficient to induce changes in mitochondrial morphology, we overexpressed it in DA neurons. Surprisingly, dPink1 overexpression also induced mitochondrial clustering (Fig. 3 A and A′). However, different from that in dPink1 mutant, the nonaggregated mitochondria appeared spherical in dPink1 overexpression neurons, and tubular structures were rarely observed. Furthermore, Drp1 overexpression (Fig. 3F) or Opa1-like heterozygosity (Fig. 3H) could not modify this dPink1 overexpression effect, suggesting that the mitochondrial aggregates in dPink1 mutant and dPink1 overexpression neurons are intrinsically different. To further verify this point, we tested the effect of Parkin overexpression in these backgrounds. Parkin overexpression can efficiently suppress dPink1 mutant phenotypes (8–10). Parkin overexpression efficiently blocked the formation of mito-GFP aggregates in dPink1 mutant (Fig. 3B), but not in dPink1 overexpression background (Fig. 3C). Interestingly, Parkin overexpression alone in wild-type background also led to a mitochondrial phenotype similar to that seen in dPink1 overexpression (Fig. 3D). To rule out the possibility that this phenotype was nonspecifically caused by overexpression, we overexpressed DJ-1a in DA neurons. No obvious effect on mitochondrial morphology was seen in this situation (Fig. S3B). The fact that mitochondrial morphology was restored by the combined actions of dParkin overexpression and dPink1 loss of function, despite the abnormal mitochondrial morphology in either condition alone, strongly suggests that the two manipulations exert opposite effects on mitochondrial dynamics. Further supporting a functional difference between the two mitochondrial states, overexpression of Pink1 or Parkin has no detrimental effect on DA neuron function (8), whereas inhibition of Pink1 does. Overexpression of Pink1 in IFM or the eye also has no effect (data not shown). A recent study showed that Pink1 overexpression induced a mild rough eye phenotype (16), the physiological relevance of which remains to be tested, because dPink1-null mutant flies do not show any eye phenotype.
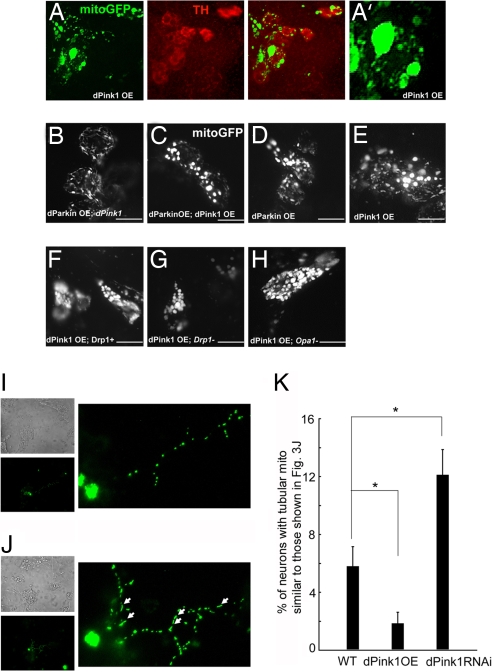
Fig. 3.
dPink1 overexpression causes mitochondrial morphological changes in DA neurons. (A) Whole-mount immunostaining of PPL1 cluster DA neurons in 5- to ≈7-day-old adult flies of the genotype TH-Gal4>UAS-mitoGFP, UAS-dPink1. (Left) Immunostaining of mitoGFP. (Center) Immunostaining of TH. (Right) Merged image. (B–H) Live imaging of mitochondria within the PPL1 cluster neurons in 5- to ≈7-day-old adult flies of the following genotypes: TH-Gal4>UAS-mitoGFP; dPink1B9; UAS-dParkin B, TH-Gal4>UAS-mitoGFP; UAS-dPink1; UAS-dParkin C, TH-Gal4>UAS-mitoGFP; UAS-dParkin D, TH-Gal4>UAS-mitoGFP; UAS-dPink1 (E), TH-Gal4>UAS-mitoGFP; UAS-dPink1; Drp1 extra copy (F), TH-Gal4>UAS-mitoGFP; UAS-dPink1; Drp1/+ (G), TH-Gal4>UAS-mitoGFP; UAS-dPink1, Opa1 like/+ (H). A′ shows higher magnification view of the mitochondrial network in A. (I and J) representative images of 1-week-old cultured wild-type fly DA neurons exhibiting punctuate-like (I) and thread-like (J) mitochondria in neuronal processes. Left images show bright-field microscopy views of the general neuronal culture (Upper) and TH+ neurons with mitochondria labeled with mito-GFP (Lower); the right images display magnified views of neuritic mitochondria of TH+ neurons. Arrows point to typical thread-like tubular mitochondria in the neurites. (K) Statistical analysis of the percentage of TH+ neurons with thread-like tubular mitochondria in their processes in the indicated genotypes. On average, ≈200 TH+ neurons for each genotype were counted. *, P < 0.01, one-way ANOVA test.
The mitochondrial aggregation phenotype seen in dPink1 overexpression background could be caused by excessive mitochondrial fission, as observed in mammalian cells overexpressing the profission protein Fis1 (17, 18). We began to test this possibility by attenuating fission via removal of one copy of Drp1 in dPink1 overexpression background, but did not observe an obvious effect (Fig. 3G). Stronger attenuation of mitochondrial fission by other means might be needed to mitigate the dPink1 overexpression effect.
To further test the activity of dPink1 in regulating mitochondrial dynamics, we analyzed mitochondrial morphology in cultured DA neurons, which allow better resolution of mitochondrial networks and units, especially in neuronal processes. In control neuronal cultures that specifically express mito-GFP in DA neurons, most neurons possess punctate, short mitochondria (Fig. 3I), and only a small number of them contain tubular mitochondria (Fig. 3J). In dPink1 mutant neuronal culture, however, there were more neurons possessing tubular mitochondria (Fig. 3K). In contrast, the number of neurons having long tubular mitochondria was significantly reduced after dPink1 overexpression (Fig. 3K). These results support the notion that dPink1 promotes mitochondrial fission.
Characterization of the Relationships Between Pink1 and Drp1 in Cultured S2R Cells.
To gain more information on the activity of dPink1 and its relationship with the fission machinery, we used Drosophila S2R cells, which allow efficient gene knockdown by RNAi. The larger size of S2R cells also allows better imaging of the mitochondrial network in the soma. In gfp dsRNA-transfected control cells, mitochondria were uniformly distributed as punctate units and tubular segments (Fig. 4A). Transfection of Drp1 dsRNA knocked down Drp1 mRNA level (Fig. S4), and caused the mitochondrial network to collapse into one large perinuclear aggregate (Fig. 4C). Transfection of dPink1 dsRNA led to a similar phenotype (Fig. 4B and Fig. S4), albeit at a lower frequency (35.6 ± 5.2% for dPink1 RNAi vs. 97.5 ± 3.6% for Drp1 RNAi). To further test the functional relationship between Drp1 and Pink1, we performed Drp1 and dPink1 double RNAi. In these cells, perinuclear aggregation of mitochondria persisted. Remarkably, a distinct phenotype not seen in single RNAi condition was observed, which was manifested as long continuous threads of mitochondria indicative of extreme fusion (27.6 ± 5.4%) (Fig. 4D). Double RNAi of Drp1 and another key mitochondrial gene MnSODII did not have the same effect (Fig. S5), suggesting that mere disruption of mitochondrial function does not lead to extreme mitochondrial fusion, and that the interaction between Pink1 and Drp1 in regulating mitochondrial morphology is specific.
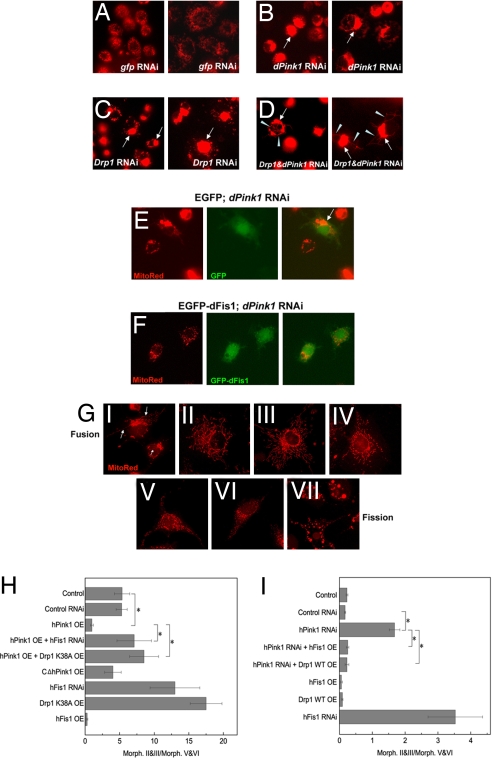
Fig. 4.
Genetic interactions between Pink1 and fission genes in modulating mitochondrial dynamics in Drosophila and mammalian cells. (A–D) Live imaging of mitochondria in S2R cells subjected to the following genetic manipulations: gfp RNAi (A), dPink1 RNAi (B), Drp1 RNAi (C), dPink1 and Drp1 double RNAi (D). Note the similar phenotype of mitochondrial aggregation (arrows) caused by dPink1 RNAi and Drp1 RNAi despite the lower percentage in the case of dPink1 RNAi. The arrowheads point to thin continuous mitochondrial tubules extending beyond mitochondrial clusters, which appeared exclusively in D. S2R cells are not homogenous in size. Two types of cells, smaller-sized (Left) and bigger-sized (Right), are shown for each genetic manipulation. The effects are similar in both sized cells. (E and F) Live imaging of mitochondria in S2R cells subject to the following genetic manipulations: dPink1 RNAi plus EGFP transfection (E); dPink1 RNAi plus EGFP-dFis1 transfection (F). EGFP-dFis1 (E) but not EGFP (F) suppressed mitochondrial clustering induced by dPink1 RNAi. The arrow marks aggregated mitochondria. (G) Seven categories of mitochondrial morphology in mammalian COS-7 cells ranging from extreme fusion (type I) to extreme fission (type VII), as revealed by MitoTracker Red staining. The categories are arbitrarily defined based on the following mitochondrial characteristics: I, perinuclear aggregates together with long continuous tubules; II, extensive, long tubular network; III, intermediate-length tubular network; IV, a mixture of short tubular mitochondria and punctate units; V, small round tubules with holes in the center; VI, small punctate dots; VII, spherical aggregates similar to that see in DA neurons overexpressing Pink1 or Parkin. To highlight the difference between categories I and VII, the long tubular mitochondria threads present in I but not VII are marked by arrows. (H and I) Statistical analysis showing that hPink1 overexpression promotes mitochondrial fission, which is prevented by the overexpression of Drp1K38A (H), whereas hPink1 RNAi promotes mitochondrial fusion in COS-7 cells, which is rescued by hFis1 or hDrp1 overexpression (I). The ratio of cells exhibiting type II and III morphology typical of fusion versus type V and VI morphology typical of fission were quantified. *, P < 0.01, one-way ANOVA test.
Pink1 Functionally Interacts with Fis1 to Regulate Mitochondrial Morphogenesis in Drosophila and Mammalian Cells.
To further examine the role of Pink1 in mitochondrial fission, we explored the functional relationship between Pink1 and Fis1, another key player in the fission pathway. Transfection of an EGFP-dFis1 fusion protein but not EGFP efficiently suppressed the mitochondrial aggregation phenotype caused by dPink1 RNAi in Drosophila S2R cells (Fig. 4 E and F) (3.6 ± 1.2% for EGFP-dFis1 vs. 41.8 ± 6.6% for EGFP). In contrast, EGFP-dFis1 was unable to suppress similar phenotypes caused by Drp1 RNAi (Fig. S6).
To test whether the functional relationship between Pink1 and Drp1/Fis1 is conserved in mammals, we used COS-7 cells, which possess dynamic mitochondrial networks (Fig. 4G and Fig. S7), and can be efficiently transfected. Overexpression of hPink1 but not hPink1ΔC resulted in more cells possessing punctate mitochondria, whereas hFis1 RNAi or overexpression of dominant-negative Drp1 (Drp1K38A) resulted in more cells having long tubular mitochondria (Fig. 4H). Overexpression of Drp1K38A in hPink1 overexpression background also resulted in more cells having long tubular mitochondria (Fig. 4H), suggesting that Drp1 may act downstream of Pink1. We then examined the effect of Pink1 knockdown in COS-7 cells. Expression of Pink1 shRNA but not a control shRNA knocked down hPink1 mRNA level (Fig. S8) and resulted in more cells having long tubular mitochondria (Fig. 4I). This effect was significantly suppressed by the overexpression of hFis1 or Drp1 (Fig. 4I). These results are consistent with Pink1, Fis1, and Drp1 being conserved positive regulators of mitochondrial fission and that Drp1 and Fis1 are epistatic to Pink1.
Discussion
Mitochondrial fission and fusion are membrane-remodeling processes that control the in vivo dynamic changes of the distribution and structure of the mitochondria network. These processes respond to energy status of the cell and are necessary for proper mitochondrial function. Dysfunction of mitochondrial fission/fusion has been linked to the pathogenesis of neurodegenerative diseases. For example, mutations in Opa-1 are associated with autosomal dominant optic atrophy (19), whereas mutations in Mfn2 cause the autosomal dominant disease Charcot–Marie–Tooth type 2A (20).
Our results demonstrate that Pink1 regulates mitochondrial morphogenesis and function through the fission/fusion pathway in IFM and DA neurons. These results link defects in mitochondrial fission/fusion to the pathogenesis of PD, a major neurodegenerative disease. A recent study reported similar genetic interactions between Pink1 and genes in the fission/fusion pathway in Drosophila, although their relationship in DA neurons, the PD-relevant cell type, was not shown in that study (16). We further show that Pink1 appears to act through Fis1 and Drp1 to regulate mitochondrial fission. Because Drp1 is recruited from the cytosol to fission sites on the outer surface of mitochondria (21, 22), whereas Pink1 is localized on the inner membrane cristae facing the intermembrane space (23), the action of Drp1 and Pink1 is likely coordinated by some intermediate protein(s) such as Fis1. Consistent with this model, direct interaction between Fis1 and Drp1 has been reported before (18). The biochemical relationship between Pink1 and Fis1 awaits further investigation. Studies in yeast have identified other proteins that interact with Drp1 or Fis1 and participate in mitochondrial fission/fusion, such as Mdv1p and Caf4p (12). Further studies will test the relationships between Pink1 and the Drosophila or mammalian counterparts of these proteins in regulating mitochondrial dynamics and DA neuron maintenance and function. Our results show that mammalian Pink1 interacts with components of the mitochondrial fission machinery and regulates mitochondrial fission/fusion, in a manner similar to that in Drosophila. This finding supports a conserved role for Pink1 in mitochondrial morphogenesis. A recent study in mammalian HeLa-7 cells showed that inhibition of Pink1 resulted in abnormal mitochondrial morphology and that this effect was rescued by Parkin overexpression (24). Intriguingly, the mitochondria in Pink1 knockdown cells appear fragmented, although some of the fragments appeared to have larger diameter. It is unclear in that case whether the phenotype was due to excessive fission or fusion. It is possible that the differential appearance of the mitochondrial network in HeLa cells and Cos-7 cells after Pink1 knockdown is due to a cell type-specific effect, or variations in the degree of Pink1 inhibition by RNAi in the two studies.
Mitochondrial fission and fusion has been implicated in cell death regulation, with Drp1-mediated fission being proapoptotic (25–27) and Mitofusin-mediated fusion being antiapoptotic (28, 29). In dPink1 mutant background, Drp1-mediated mitochondrial fission is protective. Drp1-dependent mitochondrial fission also protects against Ca2+-mediated apoptosis (30). The role of mitochondrial fission/fusion in regulating cell survival is therefore context and likely cell type dependent. This is consistent with dPink1 mutant showing phenotypes in select tissues. Neurons may be particularly vulnerable to the imbalance of mitochondrial fission/fusion because of their unique morphology and the requirement for synaptic function, in which Drp1-regulated mitochondrial fission is involved (13, 31). For example, in cultured mammalian hippocampal neurons, it has been shown that the extension or movement of mitochondria into dendritic protrusions correlates with the development and morphological plasticity of spines. Inhibition of mitochondrial fission through the expression of dominant-negative Drp1 resulted in loss of synapses and dendritic spines (31). This raises the possibility that dysfunction of Pink1 may also result in loss of dopaminergic synapses, which ultimately leads to DA neuron degeneration. In this regard, it is interesting to note that knockout of mouse Pink1 leads to impairment of dopamine release and synaptic plasticity in the striatum (32). Further studies of the regulation and function of Pink1 in synaptogenesis promise to shed light on the newly recognized role of mitochondrial dynamics in PD pathogenesis.
Materials and Methods
Drosophila Genetics and Fly Stocks.
Fly culture and crosses were performed according to standard procedures and raised at indicated temperatures. All general fly stocks and Gal4 lines were obtained from Drosophila stock centers. dPink1-null mutant line dPink1B9 was a gift from Jongkeong Chung (Korea Advanced Institute of Science and Technology, Taejon, Korea) (9). The TH-GAL4 driver was a gift from Serge Birman (Centre National de la Recherche Scientifique–Université de la Méditerranée, Marseille, France). UAS-mitoGFP was a gift from William Saxton (Indiana University, Bloomington, IN). Drp1 (or fratboy)-null mutant line drp12 and extra copy Drp1 line FLAG-FLASH-HA-drp1+ were gifts from Patrick Verstreken and Hugo Bellen (Baylor College of Medicine, Baylor, TX). The other fly stocks were described previously: UAS-dPink1 and UAS-dPink1 RNAi (8), UAS-dParkin (33), UAS-DJ-1a and UAS-DJ-1a RNAi (15), and Opa1-like mutant line P{EPgy2}CG8479 (14).
Molecular Biology.
For cell culture transfection experiment, the hPink1 and rhesus monkey Drp1 cDNAs were cloned into pCDNA3 expression vectors containing the indicated epitope tags (Invitrogen). Human full-length Pink1 (hPink1) and C-terminal truncated form (hPink1ΔC) were tagged with C-terminal FLAG epitope (8). hFis1 RNAi construct was a gift from Michael T. Ryan (La Trobe University, Melbourne, Australia) (17). hPink1 shRNA clones (ID TRCN0000007099 and TRCN0000007101) were purchased from Open Biosystems. hFis1 construct was a gift from Yisang Yoon (Mayo Clinic and Foundation, Rochester, MN) (18). Rhesus monkey wild-type Drp1 and dominant-negative Drp1k38A were gifts from E. Smirnova and A. M. van der Bliek (University of California, Los Angeles, CA) (34). dFis1 cDNA was acquired from DGRC. N-terminal EGFP was fused in-frame into dFis1 cDNA and subcloned into pAc-5.1 vector (Invitrogen). EGFP was similarly subcloned into pAc-5.1 vector, taking advantage of the built-in actin promoter. pCMV-Venus was a gift from Yuzuru Imai (Stanford University, Stanford, CA). Each construct was fully sequenced before performing transfection. dsRNAs of Drp1, dPink1, MnSODII (mitochondrial manganese superoxide dismutase), and gfp were generated according to standard protocols using in vitro transcription systems (Ambion and Epicenter). Primer sequences for individual genes are available on request. For RT-PCR analysis, total RNA was prepared by using an RNeasy kit (Qiagen). Details of the quantitative RT-PCR procedure were essentially as described in ref. 35.
Live Imaging of Mitochondrial Morphology in COS-7 Cells and S2R Cells: Visualizing Mitochondria in Fly DA Neurons.
For details of these procedures, see SI Materials and Methods. Note that to facilitate the monitoring of effects of genetic manipulations on mitochondrial fission in COS-7 cells, observation was started 2–3 h after the cells were removed from the 37°C, 5% CO2 chamber and placed at ambient temperature in the presence of MTRed dye. This treatment resulted in more control cells with tubular (or fused) mitochondria (Fig. S7), making it easier to score fission events induced by the genetic manipulations. This change in mitochondrial morphology is adaptive and transient, because when the control cells were transferred back to the 37°C, 5% CO2 chamber, their mitochondrial morphology also showed corresponding changes.
Muscle Histology and Transmission Electron Microscopy Analysis.
Muscle histology and transmission electron microscopy analysis were performed as described in ref. 33, except that Epon resin was used for embedding.
Dopamine Measurements.
HPLC analysis of catecholamine levels was performed essentially as described in ref. 15. For sample preparation, adult male fly heads were dissected out and homogenized in 0.1 M perchloric acid (generally 50 μl per four or five heads) by using a motorized hand-held tissue grinder. The homogenate was frozen immediately on dry ice and stored at −80°C before HPLC analysis.
Supplementary Material
Acknowledgments.
We are grateful to Drs. H. Bellen, S. Birman, D. Chan (Caltech, Pasadena, CA), J. Chung, M. Cookson (National Institutes of Health, Bethesda, MD), M. Fuller (Stanford University), M. T. Ryan, W. Saxton, M. Scott (Stanford University), E. Smirnova, A. M. van der Bliek, P. Verstreken, Y. Yoon, J. Zhang (Stanford University), and the Bloomington Drosophila Stock Center for fly stocks, cell lines, and molecular biology reagents. We thank Dr. S. Guo for reading the manuscript and members of the B. L. Laboratory for discussion and help. This work was supported by National Institutes of Health Grant R01AR054926 (to B.L.) and a Stanford Bio-X Graduate Fellowship (to Y.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711845105/DCSupplemental.
References
- 1.Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson's disease. Nature. 1999;399:A32–A39. doi: 10.1038/399a032. [DOI] [PubMed] [Google Scholar]
- 2.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 3.Bertoli-Avella AM, Oostra BA, Heutink P. Chasing genes in Alzheimer's and Parkinson's disease. Hum Genet. 2004;114:413–438. doi: 10.1007/s00439-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: Implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 5.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 6.Darios F, et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 7.Elkon H, et al. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J Mol Neurosci. 2002;18:229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 10.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, et al. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci USA. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 13.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 14.McQuibban GA, Lee JR, Zheng L, Juusola M, Freeman M. Normal mitochondrial dynamics requires rhomboid-7 and affects Drosophila lifespan and neuronal function. Curr Biol. 2006;16:982–989. doi: 10.1016/j.cub.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 18.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander C, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 20.Kijima K, et al. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- 21.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieu Q, Okreglak V, Naylor K, Nunnari J. The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission. J Cell Biol. 2002;158:445–452. doi: 10.1083/jcb.200205031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestri L, et al. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 24.Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 26.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 27.Abdelwahid E, et al. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 30.Szabadkai G, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–924. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.