Abstract
The trilaminate vascular architecture provides biochemical regulation and mechanical integrity. Yet regulatory control can be regained after injury without recapitulating tertiary structure. Tissue-engineered (TE) endothelium controls repair even when placed in the perivascular space of injured vessels. It remains unclear from vascular repair studies whether endothelial implants recapitulate the vascular epithelial lining or expose injured tissues to endothelial cells (ECs) with unique healing potential because ECs line the vascular epithelium and the vasa vasorum. We examined this issue in a nonvascular tubular system, asking whether airway repair is controlled by bronchial epithelial cells (EPs) or by ECs of the perfusing bronchial vasculature. Localized bronchial denuding injury damaged epithelium, narrowed bronchial lumen, and led to mesenchymal cell hyperplasia, hypervascularity, and inflammatory cell infiltration. Peribronchial TE constructs embedded with EPs or ECs limited airway injury, although optimum repair was obtained when both cells were present in TE matrices. EC and EP expression of PGE2, TGFβ1, TGFβ2, GM-CSF, IL-8, MCP-1, and soluble VCAM-1 and ICAM-1 was altered by matrix embedding, but expression was altered most significantly when both cells were present simultaneously. EPs may provide for functional control of organ injury and fibrous response, and ECs may provide for preservation of tissue perfusion and the epithelium in particular. Together the two cells optimize functional restoration and healing, suggesting that multiple cells of a tissue contribute to the differentiated biochemical function and repair of a tissue, but need not assume a fixed, ordered architectural relationship, as in intact tissues, to achieve these effects.
Keywords: tissue engineering, airway disease, vascular disease
Control of vascular homeostasis after injury can be regained without restoring normal and complex vascular architecture. Endothelial cells (ECs) embedded within a 3D denatured collagen matrix secrete antiproliferative, antithrombotic, and antiinflammatory agents that promote vascular repair even when such constructs are placed far from the lumen within the vascular adventitia. Luminal inflammation, occlusive thrombosis, and intimal hyperplasia can be controlled by allo- and xenogeneic matrix-embedded (ME) ECs in a manner indistinct from native endothelium and without eliciting a significant immune response (1–8). The challenge posed is whether endothelial implants recapitulate the endothelium lining as an epithelium or add to the regulation by perfusing vessels of the vasa vasorum, which are principally comprised of ECs. This question, however, cannot be elucidated in a blood vessel where ECs of vasa vasorum and vascular epithelium are one and the same. In all other physiological tubes, the ECs still comprise the perfusing microvasculature, but a more distinct cell serves as an epithelial lining. Structural similarities and differences between blood vessels and other tubular physiological structures provide an opportunity to investigate how ME cells mediate repair.
As with blood vessels, the airways, intestines, ureters, and fallopian tubes possess a trilaminate form that offers structural integrity and flow modulation, as well as profound regulation of local inflammation, thrombosis, hemostasis, proliferation, and remodeling (9–11). In these structures, a loose fibrous adventitia is replete with coursing arterioles, venules, and neural forms; a middle muscularis layer provides control of luminal dimensions and tone; and a surface layer contains cells that biochemically regulate the wall response, sense flow, and luminal contents.
The airway epithelium, like the vascular endothelium, was once thought to be only a physical barrier between the inner tissues of the respiratory system and the external environment. It is now recognized that the epithelium regulates an array of airway functions, including control of lung fluid balance, attraction and activation of inflammatory cells, metabolism and clearance of inhaled agents, and regulation of airway smooth muscle function. As with the vascular endothelium, the airway epithelium exerts this control through the diverse array of compounds it can produce, such as growth factors, airway-constricting peptides, chemokines, cytokines, gases, and lipid mediators (9, 12). In airway disorders, such as asthma and chronic obstructive pulmonary disease (COPD), a functional imbalance is tightly associated with remodeling of airway structure. Remodeling can establish an abnormal microenvironment between the epithelium and underlying mesenchyme, finally resulting in a pathophysiological cycle of inflammatory injury and aberrant repair (13, 14). These alterations in airway remodeling recapitulate features in the development of neointima formation, where endothelium injury initiates a repair response that can lead to luminal narrowing and diminished vessel function (15).
In the present article, we used an in vivo airway injury model and cell culture models to understand whether injury and repair of the airway are mediated by the first-line sensor, the airway epithelium, or by the ECs of the perfusing vasculature. TE implants of the bronchial epithelium and endothelium promoted specific and synergistic repair of the airway through biochemical regulation of the airway microenvironment. Whereas epithelial cells (EPs) modulate local tissue composition and reaction, ECs preserve the epithelium and perfusing microvasculature; together, their relative impact was enhanced, suggesting both cell types are required for optimal airway repair. From these findings, it may be inferred that, in vascular injury, engineered EC implants recapitulate aspects of ECs from both the epithelium and perfusing microvasculature, whereas airways and other tubular organs likely require distinct EP and EC implants to achieve the same degree of repair. In all of these cases, healing does not require the reconstitution of architecture.
Results
Growth Kinetics of ME Cells.
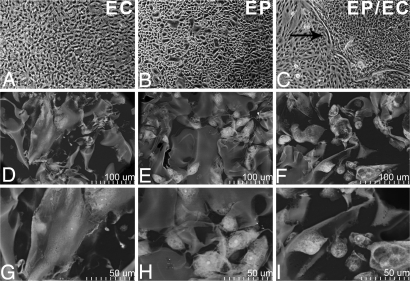
Normal human bronchial EPs and human aortic ECs were grown to confluence on tissue culture polystyrene (TCPS) and to growth arrest in denatured collagen matrices. The morphology of ECs (Fig. 1A) and EPs (Fig. 1B) on TCPS were distinct; the former exhibit a tight cobblestone appearance, whereas the latter appear as smaller, cuboidal basal cells interspersed with larger, squamous mucous cells. Cell growth on denatured collagen-coated TCPS (DCCT) was identical to growth on TCPS alone. As on TCPS, the morphology of ME-ECs (Fig. 1 D and G) and ME-EPs (Fig. 1 E and H) were distinct. ECs and EPs attained planar confluence on TCPS, but in less constrained forms in matrices. Embedded ECs retained a cobblestone appearance, and EPs appeared in their native cuboidal basal and squamous mucosal forms. Interestingly, from visual observation, cocultures of EPs and ECs on TCPS seemed to remain in isolated single-cell colonies separated by an extracellular matrix barrier, whereas the cell types were interspersed in the matrices (Fig. 1C). To obtain similar growth kinetics over 28 days, 2 × 105 EPs or 0.9 × 105 ECs were initially seeded in the matrices (2.5 × 1.0 × 0.3 cm3) [supporting information (SI) Fig. S1]. Cells' density peaked at 12–14 days and plateaued thereafter. Cell viability over a 28-day culture period, as assessed by trypan blue exclusion, averaged 91.9 ± 0.47% for embedded EPs, 92.6 ± 0.33% for ECs, and 91.2 ± 0.19% with both cells present.
Fig. 1.
Morphology of EPs, ECs, and EPs/ECs grown on TCPS and with denatured collagen matrices. (A–C) Matrix embedding allows cells to attain a conformation not limited to planar restrictions imposed by TCPS or the apparently distinct isolation of cell types and interposing barrier (black arrow). (Magnification: ×40.) (D–I) Embedded cells conform to the architecture and surface of the local struts of matrix pores in a lesser strained configuration. [Magnification: ×400–450 (D–F) and ×900 (G–I).]
Biosecretory Activity of ME Cells.
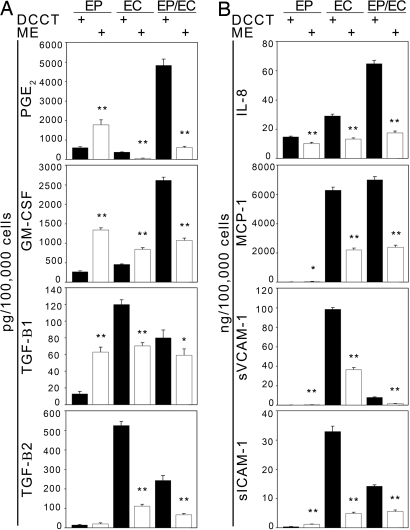
The EP and EC production of biosecretory factors involved in airway injury and repair (9, 12) was similarly dependent on matrix embedding and the presence of complementary cell type. In comparison with TCPS or DCCT cell seeding, prostaglandin E2 (PGE2) and TGFβ1 were elevated in ME-EPs and lower for ME-ECs. GM-CSF was increased in both embedded EPs and ECs, and TGFβ2 was reduced in ME-EC. In contrast, when EPs and ECs resided together in the matrices, all molecules examined were down-regulated with or without TNFα stimulation (Fig. 2A and Fig. S2). This effect extended to the expression of proinflammatory molecules. IL-8 release was significantly lower in all ME cells compared with growth on TCPS and DCCT (Fig. 2B and Fig. S3). Monocyte chemoattractant protein (MCP)-1 production was reduced significantly in embedded ECs and EPs/ECs, compared with tissue culture plate conditions. EPs expressed relatively low levels of MCP-1 compared with ECs and EPs/ECs for all conditions. The soluble form of ICAM-1 (sICAM-1) was significantly reduced in embedded ECs and EPs/ECs and significantly increased in ME-EPs, compared with tissue culture plate conditions; however, EP production of sICAM-1 was relatively low compared with ECs and EPs/ECs. Embedded EC and EP/EC release of the soluble form of VCAM-1 (sVCAM-1) was significantly decreased compared with tissue culture plate conditions, whereas EP release was significantly higher. Similar to EP expression of sICAM-1, EP expression of sVCAM-1 was relatively low compared with ECs and EPs/ECs for all conditions.
Fig. 2.
Protein secretion levels vary depending on cell type and substrate. (A) Protein levels of PGE2, GM-CSF, TGFβ1, and TGFβ2 secreted by EPs, ECs, and EPs/ECs grown on DCCT and matrices were substratum and cell-specific. Cells were grown to confluence and stimulated with 10 ng/ml TNFα for 24 h. (B) Expression of proinflammatory chemokines (IL-8 and MCP-1) and adhesion molecules (sICAM-1 and sVCAM-1) is down-regulated in embedded cells stimulated with 10 ng/ml TNFα for 24 h. *, P < 0.05; **, P < 0.001 vs. DCCT in the same cell type (n = 5–6).
EP and EC Implants Differentially Regulate Airway Repair.
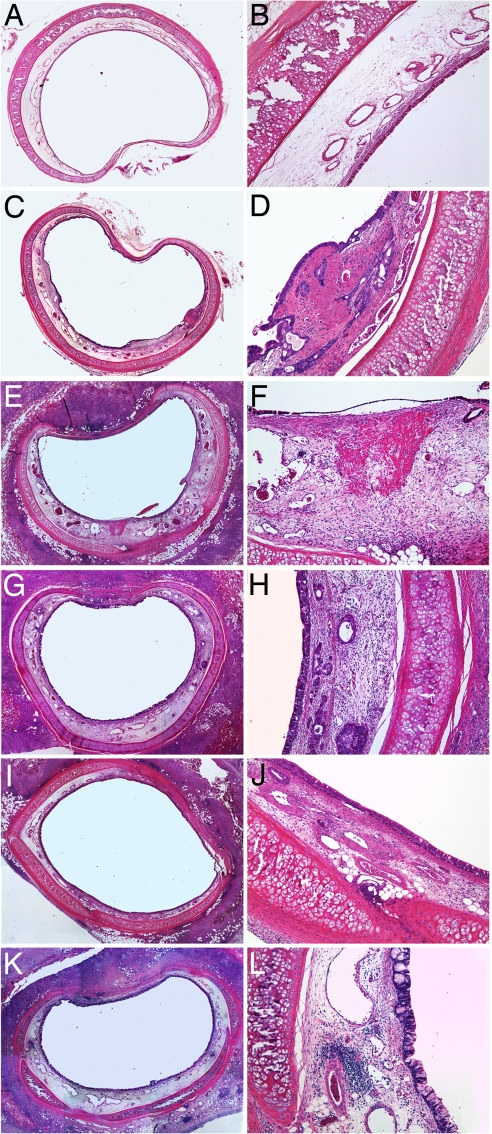
Twenty-nine rabbits underwent controlled airway injury and randomly received no further therapy, acellular matrices, or matrices with ECs or EPs alone or together. Brush injury induced reproducible tracheal epithelial denudation over 30% of the surface area and with significant thinning elsewhere. Such injury resulted in luminal narrowing, mesenchyme cell hyperplasia, collagen deposition, hypervascularity, and infiltration of inflammatory cells (Fig. 3). Tissue effects were mirrored on a cellular level. Extensive cell damage and airway remodeling were noted in the absence of further intervention (Fig. 3 C and D), compared with control uninjured airways (Fig. 3 A and B), and unaffected by matrices alone (Fig. 3 E and F). ME cells enhanced repair in a distinct manner when alone and synergistically when cultured together (Fig. 3 G–L).
Fig. 3.
Peritracheal EP and EC grafts regulate airway injury 9 days after epithelial denuding. (A–F) Injury-induced mesenchymal hyperplasia (C and D) compared with intact controls (A and B) and the acellular implants induced modest peribronchial inflammation (E and F) with more extensive tissue damage, mesenchymal hyperplasia, neovascularization, and luminal narrowing. (G–L) Embedded ECs (G and H) or EPs (I and J) alone reduced these effects and optimally when both cells were present in the constructs (K and L). (Magnification: A, C, E, G, I, and K, ×20; B, D, F, H, J, and L, ×40.)
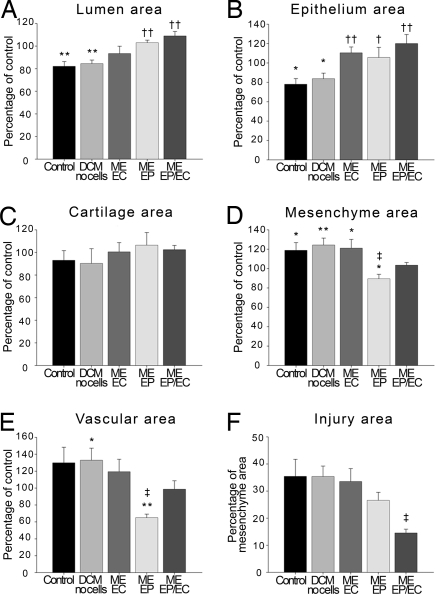
Luminal obstruction and airway remodeling were controlled by ME-EP, alone and with ME-ECs, and, to a lesser extent, isolated ME-ECs (Figs. 4 and 5). The area of the airway lumen was reduced with injury (−17.9 ± 4.2%, compared with uninjured control segments). Whereas acellular matrices had no effect on this injury, the lumen was preserved in major part by ME-EC, with a 62.5 ± 6.5% reduction in luminal occlusion, and dilated to a degree by embedded EPs (3.0 ± 2.3%) and even greater with ME of both cells together (8.9 ± 4.0%) (Fig. 5A). Epithelial area was reduced 22.0 ± 5.9% by injury and was unaffected by acellular implants. Epithelial area was best preserved in tracheas wrapped with embedded ECs alone (110.5 ± 6.1%) or with EPs (120.1 ± 9.3%) but to lesser extent with EPs (105.6 ± 10.5% (Fig. 5B). Epithelial surface area was similarly greatest in the combined cell group at 80.9 + 3.9%, but in no specimen was complete epithelial recovery observed. The values for total area reflect the complex epithelial structure that, unlike the monolayer of the vascular endothelium, has a defined thickness. There was no difference in cartilage area among all of the conditions (Fig. 5C). Mesenchymal hyperplasia was evident after injury as an 18.9 ± 8.0% increase in thickness and was made, if anything, subtly worse with empty matrices (124.3 ± 7.2%). EC implants had no affect on the mesenchyme, and EP matrices reduced thickening by 40.0 ± 1.8% and virtually eliminated hyperplasia altogether with implants containing ECs and EPs (Fig. 4D). Injured airways were hypervascular with 29.9 ± 18.5% greater vessel density and marginally worse when wrapped with acellular matrices, 32.9 ± 14.4%. Injured airways were marginally better with embedded ECs, significantly reduced by EPs (64.9 ± 4.4%), and relatively normal in matrices with both cells (Fig. 5E). Tissue injury as a percentage of mesenchyme area was 2.4-fold reduced in tracheas wrapped with EPs and ECs (14.5 ± 1.4%), compared with no implants, empty implants, or implants with ECs (Fig. 5F). Embedded EPs had a midrange effect with a 24.9 ± 0.75% reduction in injury.
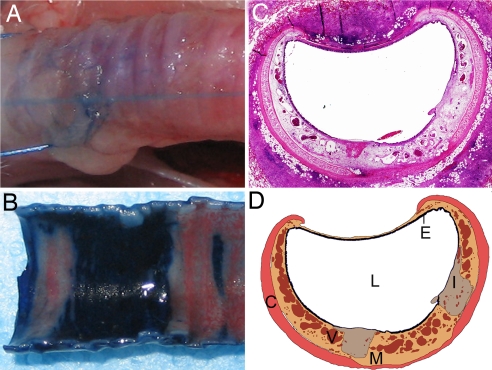
Fig. 4.
Tracheal epithelial injury is localized in the airway injury model. (A and B) Trypan blue is excluded from deposition in the bronchial wall by an intact epithelium (A) but only in areas of remnant epithelium after localized nylon brush injury (B). Areas of epithelial denudation are demarcated by dark blue infiltration. (C and D) Histological cross-sections of rabbit trachea wrapped with a denatured collagen matrix 9 days after injury (C) were digitally reconstructed (D) for quantification of the areas of the lumen (L), epithelium (E), mesenchyme (M), cartilage (C), vascular (V), and injured tissue (I). (Magnification: C, ×20.)
Fig. 5.
EP and EC implants promote tracheal healing in a rabbit airway. Although both cell types promote epithelial recovery, EPs protect mesenchymal hyperplasia and prevent neovascularization. Together, the two cells optimize repair. (A–E) Areas measured were lumen (A), epithelium (B), cartilage (C), mesenchyme (D), and vascular (E). (F) Area of tissue injury expressed as percentage of mesenchyme area. Data expressed as mean percentage ± SE of control (noninjured) values from rabbits receiving implants with EPs and ECs (n = 4), only EPs (n = 5), only ECs (n = 7), no cells (n = 7), or no implants (n = 6). *, P < 0.05; **, P < 0.01 vs. control; †, P < 0.05; ††, P < 0.01 vs. acellular matrices and no implants. ‡, P < 0.05 vs. acellular devices, no implants, or embedded ECs.
As the matrix implants degrade, they allow for moderate and controlled inflammatory cell infiltration. This finding was evident in all sections and was no different in implants with and without cells. Similarly, tissue inflammation was identical with or without implanted matrices. The extent of fibrosis and necrosis was less in tracheas wrapped with matrices containing EPs alone or with ECs. The incidence of stridor in the rabbits was recorded 9 days after brush injury. Only one of six (17%) rabbits that did not have implants exhibited signs of stridor, whereas stridor was presented in five of seven (71%) rabbits treated with acellular devices. Interestingly, the incidence of stridor in rabbits treated with ME-ECs was only two of seven (29%), whereas no signs of stridor were observed in the five rabbits treated with ME-EPs or the four rabbits treated with dual-cell implants.
Fibroblast Growth Inhibited by ME Cells.
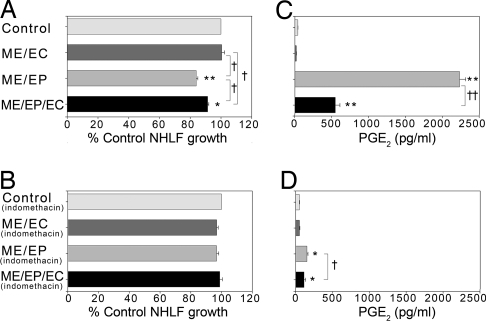
Fibroblasts are an essential supporting cell of the airway and the mesenchymal hyperplastic lesion. Normal human lung fibroblast (NHLF) proliferation was maximally reduced when cocultured with matrices containing EPs alone, modestly reduced by embedded EPs and ECs, and unaffected by ME-EC cocultures. The EP effect depended on prostaglandin production. Then, 5 μM indomethacin aborted fibroblast growth inhibition (Fig. 6 A and B), and production of PGE2, a potent inhibitor of fibroblast proliferation (16–18), inversely correlated with NHLF growth in our coculture system (R2 = 0.95) (Fig. 6C). PGE2 levels were highest (2,231.7 ± 81.1 pg/ml) in NHLF cocultured with ME-EPs, rising >50-fold above that seen with fibroblasts alone (40.2 ± 2.0 pg/ml). In contrast, ME-ECs reduced PGE2 levels 2-fold (20.4 ± 1.8 pg/ml) when alone and limited the EP increase 4-fold (549.2 ± 60.7 pg/ml). Indomethacin significantly diminished PGE2 production in both NHLF cultures with embedded EPs or EPs/ECs (Fig. 6D).
Fig. 6.
PGE2 expression in cocultures of NHLFs and embedded cells inversely correlates with NHLF growth. (A) NHLF growth is reduced in cocultures with EPs embedded alone or with ECs. (B) NHLF growth inhibition is reversed in the same cocultures after 48-h exposure to 5 μM indomethacin. (C) PGE2 levels were higher in NHLF cocultures with EPs embedded alone or with ECs. (D) PGE2 production was significantly diminished after 48-h exposure to 5 μM indomethacin. *, P < 0.00001; **, P < 0.000001 vs. control; †, P < 0.05; ††, P < 0.00001 vs. compared condition (n = 4).
Tube Formation Inhibited by ME Cells.
The effect of ME cells on angiogenesis in vitro was examined by tube formation of human umbilical vein endothelial cells (HUVECs) cultured with conditioned medium. This process involved migration, invasion, and differentiation of HUVECs in the formation of the tubular network (Fig. S4A) (19, 20). Tube length and branch point numbers were highest with embedded ECs and lowest with embedded EPs, and together the cells matched acellular controls (Fig. S4 B–D). Tube formation correlated with FGF-2 production by embedded cells (R2 = 0.95) (Fig. S4 E and F).
Discussion
Unlike blood vessels in which the ECs serve as both the central element of the epithelium and vasa vasorum, the airway epithelium is composed of highly differentiated cells that are quite distinct from the ECs that line the bronchial vasorum. This cellular distinction in the airway allowed for an investigation of the differential control these two cell systems may provide in mediating tissue repair. We found that TE implants of the bronchial epithelium and endothelium promoted specific and synergistic repair of the airway after controlled, quantifiable injury through unique biochemical regulation of the airway microenvironment. It appears that, although epithelial cells limit tissue injury and mesenchymal hyperplasia, ECs promote preservation of the epithelium and perfusion of the injured tissue. Moreover, each effect is augmented in the presence of its complementary cell type, and overall repair is optimal when both cells are presented to the injured airway.
EPs and ECs display identical growth kinetics when embedded in denatured collagen matrices (Fig. S1). Matrix embedding enabled cell growth with less strained conformational molding to substratum, without imposing planar restraint, and retention of distinct morphology and secretory ability even without complete contiguity. In contrast to the morphology observed on TCPS and DCCT, coembedded ECs and EPs are interspersed with no apparent interceding physical barrier (Fig. 1).
The functional impact of these cellular differences was studied in a model of controlled tracheal injury. Embedded EPs and/or ECs were implanted in the tracheal adventitial space external to the site of denuding bronchial epithelial injury. Localized brush injury to rabbit tracheal epithelium produced stridor and pathological characteristics that often accompany airway diseases such as asthma and COPD. These changes include luminal narrowing, epithelial damage, mesenchymal cell hyperplasia, and hypervascularity (Fig. 3 A–F) (9, 12, 21–23). Peribronchial cuffing with implanted matrices may exacerbate injury as in other wrapped organs (24). Airflow was sufficiently restricted by combined brush denudation from within and acellular matrices from without to increase stridor >4-fold compared with injury alone. The response to our airway injury is reminiscent of inflammatory and proliferative reactions to vascular endothelial denudation that result in intimal hyperplasia, luminal narrowing, and diminished vessel function (15). As in blood vessels in which ME-ECs restored function well before restoring architectural integrity, embedded EPs or EPs/ECs preserved airway health and enhanced repair before restoring airway structure. Biochemical production, morphological effects, and functional benefit were all optimized by dual cell implants and without the need to completely rebuild the injured airway.
Remodeling in airway diseases includes major structural and functional changes to the mesenchyme, along with angiogenesis and microvascular remodeling. Larger, more abundant, and dysfunctional blood vessels are formed with uncontrolled permeability, leukocyte adhesion, and local inflammation (23, 25–27). The impact of ME-EP extended into these domains as well, restricting the hypervascular mesenchymal response to injury, but to such an extent that this layer was perhaps underperfused (65% of control). Tracheobronchial segments treated with ME-ECs were hyperplastic (121% of control) and hypervascular (119% of control), no different from untreated tissue. However, together, ECs and EPs achieved the ideal set point, maximizing all indices of repair by enhancing luminal and epithelium areas, inhibiting mesenchymal hyperplasia and hypervascularity, and significantly reducing the extent of tissue injury (Figs. 4 and 5).
A powerful bidirectional communication exists between the epithelium and the subepithelial mesenchyme. Repairing epithelium produces factors that directly influence subepithelial fibroblast migration, remodeling, and differentiation into myofibroblasts. In turn, these underlying cells release growth factors that promote autocrine proliferation and paracrine effects on blood vessels, smooth muscle, and the epithelium (9, 21). EPs and ECs cultured separately or together secrete different patterns of airway-repair and injury-specific molecules on TCPS or DCCT, and these cellular secretion levels are modulated significantly further when these cells are matrix embedded (Fig. 2 and Figs. S2 and S3) (9, 12, 21). All molecules studied were down-regulated in coembedded EPs and ECs with or without TNFα stimulation. Whereas ME-EP express higher levels of PGE2, GM-CSF, and TGFβ1, ME-ECs secrete lower levels of PGE2, TGFβ1, and TGFβ2 compared with cells on TCPS and DCCT. The diverse expression patterns among EPs, ECs, and EPs/ECs grown on TCPS, DCCT, or in matrices may arise from complex cellular, biochemical, and substrate-specific interactions regulating cellular secretion.
The secretion of mediators of vascular and bronchial biology may attain different local levels and act differently when both ECs and EPs are present together rather than alone. For example, PGE2 is a potent bronchodilator that stabilizes extracellular matrix in the airway (16, 28) and inhibits fibroblast proliferation (16, 18). Yet, this same molecule also induces vascular permeability and edema (29). Therefore, the influence of PGE2 on bronchial injury might well be appreciated differentially by EPs or ECs alone or the two cells together. Indeed, ME-EP inhibition of NHLF growth correlated with the production of PGE2, and both were reversed by the cyclooxygenase 1 and 2 inhibitor indomethacin. FGF-2 has similar potentially competing organ-specific functions. This growth factor is a potent vascular endothelial mitogen and angiogenic factor (25) but also stimulates bronchial smooth muscle cell proliferation and airway inflammation (30). Therefore, it is fascinating that, although ME-EPs produce the lowest amount of FGF-2 and reduce tube formation in vitro and mesenchymal vascularization in vivo, the reverse is true for ECs. ME-ECs modestly enhance tube formation and branching in vitro in concert with elevated FGF-2 (Fig. S4E) and stimulate neovascularization to a small degree after airway injury. The most balanced effect is seen when the two cells are together. Vascular density is restored to control states in vivo and branching and tube formation are restored to control levels in vitro.
Local inflammation seems dominated by endothelial biology, and endothelial immunogenicity and interaction with immune cells are governed by cell matrix interconnections. The immunological response, in previous studies, to allogeneic and even xenogeneic ECs was muted and even reduced the minimal inflammatory cell infiltration that accompanies the controlled pace of matrix degradation (4, 6, 8). ME-ECs express decreased levels of MHC class II, costimulatory, chemokine, and adhesion molecules compared with planar ECs (1–4, 31). We now show significantly reduced expression of IL-8, MCP-1, sICAM-1, and sVCAM-1 by embedding of ECs alone or with EPs. ME-EPs produce lower levels of IL-8 compared with TCPS and DCCT levels but more sICAM-1 and sVCAM-1; all of these levels are relatively low compared with ECs alone or EPs and ECs together. We have not previously reported embedding of a cell type other than ECs altering expression levels of proinflammatory molecules. These results, along with our previous findings, raise questions as to whether altered molecule expression and reduced cell immunogenicity of embedded cells arise from cell attachment alone or only when combined with a specific supporting substratum. The peribronchial infiltration of inflammatory cells in xenogeneic-embedded EC and EP implants was moderate and indistinguishable from acellular matrices.
Tissue engineering provides a platform to differentiate the distinct effects of different cells alone and together on local tissue repair. Two of the major cells resident in the airway, the ECs and EPs, offer synergistic healing linked to their unique biochemical function. The EPs that serve as local biosensors and highly differentiated secretory cells have the greatest range of effect, whereas the ECs, which comprise nourishing vessels, aid in preservation of the epithelium and perfusing microvasculature. It is becoming evident that the EPs may well provide for functional control of organ injury and fibrous response, and ECs may well provide for preservation of the tissue perfusion, and the epithelium in particular. Together, the two cells optimize functional restoration and healing. In vascular injury, embedded ECs may enable vascular repair through simultaneous recapitulation of two regulatory units, the vascular endothelium as an epithelium and ECs in arteries of the vasa vasorum. The airway and other tubular organs, however, likely require the intact function of their distinct epithelial cells as well as ECs to achieve the same degree of repair, and in none of these cases is recapitulation of the final ordered architecture an absolute necessity for healing.
Methods
Cell Culture and Cell Engraftment.
EPs were grown in bronchial epithelial growth medium (BEGM), ECs and HUVECs were grown in endothelial growth medium-2 (EGM-2) supplemented with 10% FBS, and NHLFs were grown in fibroblast growth medium supplemented with 5% FBS. When EPs and ECs were cocultured, a 1:1 mixture of BEGM:EGM-2 was used. EPs and ECs also were grown together or separately on Gelfoam (Amersham Pharmacia/Upjohn), a denatured collagen matrix.
Animals and Surgical Procedure.
A modified version of a rabbit airway injury model (32) recapitulated acute and chronic changes of airway structure and function. A long midline incision was made exposing the trachea. A 2-mm nylon brush inserted through an endotracheal tube was advanced and withdrawn across a defined area of the trachea 10 times, inducing a localized and locatable injury of the epithelial lining. The injured site was then wrapped with matrices containing no cells, ECs, EPs, or EPs/ECs. Injured and untreated animals served as controls. Localized nylon brush injury of rabbit tracheal epithelium was confirmed with trypan blue dye transport (Fig. 4 A and B).
Tissue Processing and Analysis.
After removal, the tracheas were fixed in 10% formalin and sectioned into 3-mm-long segments. Five-micrometer sections were obtained from the proximal, middle, and distal segments and stained with H&E and verHoeff's elastin stain. The degree of airway injury was determined by morphometric analysis (Fig. 4 C and D).
Biosecretory Characterization.
The biosecretory function of ECs and EPs in denatured collagen matrices, TCPS, and DCCT, were compared. TNFα was used as a stimulant given its inflammatory and cytotoxic involvement in a broad range of pulmonary diseases (33). After 24 h, the medium was collected and analyzed by using commercially available ELISA kits.
Fibroblast Cocultures.
NHLFs were seeded at 5 × 104 cells per well and grown for 48 h on six-well transwell plates (Costar). Fresh medium (5 ml per well of BEGM:EGM-2, 1:1) was then added with or without 5 μM indomethacin (Sigma), and 24-mm inserts (0.4-μm membrane) with matrices (engrafted with ECs, EPs, or EPs/ECs) were placed on top of the NHLFs. After an additional 48 h, NHLFs were trypsinized, and the total cell number for each well was determined with a cell counter (Coulter). Medium was collected and analyzed by using commercially available ELISA kits.
In Vitro Tube-Formation Assay.
A CAS HT tube formation kit (Trevigen) was used. HUVECs were plated at a density of 2 × 104 cells per well and incubated at 37°C for 8 h with either BEGM:EGM-2 (1:1) medium unconditioned (incubated at 37°C without cells for 48 h) or conditioned for 48 h from matrices engrafted with ECs, EPs, or EPs/ECs. Images from four microscopic fields per well were analyzed by using Adobe Photoshop CS3 software.
Data and Statistical Analysis.
All data are expressed as means ± SE. All statistical analysis was performed by using Statistica 6.0 software. Data between two groups were analyzed by Student's t test. Data among more than two groups were evaluated by ANOVA, followed by Fisher's least significant difference post hoc test to assess intergroup significance. A value of P < 0.05 was considered significant.
Additional Details.
For a more detailed description of the methods, see SI Methods.
Supplementary Material
Acknowledgments.
We thank M. Chambers for extensive technical assistance and animal care; A. C. Paz, J. Pettit, P. Seifert, and B. Yang for valuable technical assistance; and E. Ingenito, E. Nugent, W. Wagner, and S. Hess for comments on the article. This work was supported by National Institutes of Health Grant R01 GM 49039.
Footnotes
Conflict of interest statement: The authors have a pending patent on the technology presented in this article that has been licensed to Pervasis Therapeutics. R.L. and E.R.E. hold an equity share in this company.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802463105/DCSupplemental.
References
- 1.Methe H, Edelman ER. Tissue engineering of endothelial cells and the immune response. Transplant Proc. 2006;38:3293–3299. doi: 10.1016/j.transproceed.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Methe H, Edelman ER. Cell-matrix contact prevents recognition and damage of endothelial cells in states of heightened immunity. Circulation. 2006;114:I233–I238. doi: 10.1161/CIRCULATIONAHA.105.000687. [DOI] [PubMed] [Google Scholar]
- 3.Methe H, Groothuis A, Sayegh MH, Edelman ER. Matrix adherence of endothelial cells attenuates immune reactivity: Induction of hyporesponsiveness in allo- and xenogeneic models. FASEB J. 2007;21:1515–1526. doi: 10.1096/fj.06-7051com. [DOI] [PubMed] [Google Scholar]
- 4.Methe H, et al. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112:I89–I95. doi: 10.1161/01.CIRCULATIONAHA.105.524991. [DOI] [PubMed] [Google Scholar]
- 5.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci USA. 1995;92:8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugent HM, Edelman ER. Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J Surg Res. 2001;99:228–234. doi: 10.1006/jsre.2001.6198. [DOI] [PubMed] [Google Scholar]
- 7.Nugent HM, et al. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: A safety and efficacy study in the pig. J Vasc Res. 2002;39:524–533. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 8.Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ Res. 1999;84:384–391. doi: 10.1161/01.res.84.4.384. [DOI] [PubMed] [Google Scholar]
- 9.Knight DA, Holgate ST. The airway epithelium: Structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 10.Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- 11.Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 12.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- 13.Hicks W, Jr, et al. Does cartilage down-regulate growth factor expression in tracheal epithelium? Arch Otolaryngol Head Neck Surg. 1999;125:1239–1243. doi: 10.1001/archotol.125.11.1239. [DOI] [PubMed] [Google Scholar]
- 14.Holgate ST, et al. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–98. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AG. Emigration and immigration of mesenchymal cells: A multicultural airway wall. Eur Respir J. 2004;24:515–517. doi: 10.1183/09031936.04.00067404. [DOI] [PubMed] [Google Scholar]
- 16.Charbeneau RP, et al. Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/− mice: Implications for fibroproliferation. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1103–L1111. doi: 10.1152/ajplung.00350.2002. [DOI] [PubMed] [Google Scholar]
- 17.Pan T, Mason RJ, Westcott JY, Shannon JM. Rat alveolar type II cells inhibit lung fibroblast proliferation in vitro. Am J Respir Cell Mol Biol. 2001;25:353–361. doi: 10.1165/ajrcmb.25.3.4004. [DOI] [PubMed] [Google Scholar]
- 18.Moore BB, et al. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284:L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 19.Yue PY, et al. The angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Tjiu JW, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–1151. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]
- 21.Knight D. Epithelium-fibroblast interactions in response to airway inflammation. Immunol Cell Biol. 2001;79:160–164. doi: 10.1046/j.1440-1711.2001.00988.x. [DOI] [PubMed] [Google Scholar]
- 22.Munakata M. Airway remodeling and airway smooth muscle in asthma. Allergol Int. 2006;55:235–243. doi: 10.2332/allergolint.55.235. [DOI] [PubMed] [Google Scholar]
- 23.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 24.Huth F, Kojimahara M, Franken T, Rhedin P, Rosenbauer KA. Aortic alterations in rabbits following sheathing with silastic and polyethylene tubes. Curr Top Pathol. 1975;60:1–32. doi: 10.1007/978-3-642-66215-7_1. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M, Tanaka H, Abe S. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest. 2005;127:965–972. doi: 10.1378/chest.127.3.965. [DOI] [PubMed] [Google Scholar]
- 26.Horvath G, Wanner A. Inhaled corticosteroids: Effects on the airway vasculature in bronchial asthma. Eur Respir J. 2006;27:172–187. doi: 10.1183/09031936.06.00048605. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JW, Kotsimbos T. Airway vascular remodeling in asthma. Curr Allergy Asthma Rep. 2003;3:153–158. doi: 10.1007/s11882-003-0028-3. [DOI] [PubMed] [Google Scholar]
- 28.Chung KF. Evaluation of selective prostaglandin E2 (PGE2) receptor agonists as therapeutic agents for the treatment of asthma. Sci STKE. 2005;2005:pe47. doi: 10.1126/stke.3032005pe47. [DOI] [PubMed] [Google Scholar]
- 29.Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol. 1984;2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 30.Bosse Y, Rola-Pleszczynski M. FGF2 in asthmatic airway-smooth-muscle-cell hyperplasia. Trends Mol Med. 2008;14:3–11. doi: 10.1016/j.molmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Methe H, Hess S, Edelman ER. Endothelial immunogenicity—A matter of matrix microarchitecture. Thromb Haemost. 2007;98:278–282. [PubMed] [Google Scholar]
- 32.Nakagishi Y, et al. Rabbit model of airway stenosis induced by scraping of the tracheal mucosa. Laryngoscope. 2005;115:1087–1092. doi: 10.1097/01.MLG.0000163105.86513.6D. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.