Abstract
The human protein Cox17 contains three pairs of cysteines. In the mitochondrial intermembrane space (IMS) it exists in a partially oxidized form with two S–S bonds and two reduced cysteines (HCox172S-S). HCox172S-S is involved in copper transfer to the human cochaperones Sco1 and Cox11, which are implicated in the assembly of cytochrome c oxidase. We show here that Cu(I)HCox172S-S, i.e., the copper-loaded form of the protein, can transfer simultaneously copper(I) and two electrons to the human cochaperone Sco1 (HSco1) in the oxidized state, i.e., with its metal-binding cysteines forming a disulfide bond. The result is Cu(I)HSco1 and the fully oxidized apoHCox173S-S, which can be then reduced by glutathione to apoHCox172S-S. The HSco1/HCox172S-S redox reaction is thermodynamically driven by copper transfer. These reactions may occur in vivo because HSco1 can be found in the partially oxidized state within the IMS, consistent with the variable redox properties of the latter compartment. The electron transfer-coupled metallation of HSco1 can be a mechanism within the IMS for an efficient specific transfer of the metal to proteins, where metal-binding thiols are oxidized. The same reaction of copper–electron-coupled transfer does not occur with the human homolog of Sco1, HSco2, for kinetic reasons that may be ascribed to the lack of a specific metal-bridged protein–protein complex, which is instead observed in the Cu(I)HCox172S-S/HSco1 interaction.
Keywords: cytochrome c oxidase assembly, copper chaperone, mitochondrial chemistry, NMR
Copper is an essential element for living organisms, in which it is required by a number of proteins responsible for a wide range of biological processes, including mitochondrial respiration (1). Specific mechanisms, involving a battery of proteins, are necessary for living organisms for safely and efficiently delivering copper ions from their entry in the cell to its final protein incorporation (2).
Cytochrome c oxidase (CcO), the terminal oxidase of the respiratory chain, needs three copper ions to perform its function (3). The CcO enzyme in mammals is located within the mitochondrial inner membrane and is constituted by 13 subunits, two of which contain two copper centers (4). One, called CuB, is a monocopper site in subunit I, and the other, called CuA, is a binuclear site in subunit II (4). A number of proteins are required for the metallation of these sites (5). For copper insertion into the CuA site, Cox17, Sco1, and Sco2 proteins have been proposed to have essential nonoverlapping roles within the mitochondrial intermembrane space (IMS), the first working as a metallochaperone to Sco1 and Sco2 (6, 7), whereas the other two have a cochaperone function during the copper insertion in the CuA site (7). Accordingly, lack of Cox17 or mutations on either Sco1 or Sco2 produce pathogenic conditions related to decreased CcO activity with fatal outcomes in mice and humans, respectively (8, 9). Also, yeast lacking Cox17 is respiratory deficient because of the lack of CcO activity, and this mutant phenotype is suppressed by the addition of 0.4% copper salts to the growth medium (10).
Human Cox17 (HCox17) is a 62-residue protein and contains six conserved Cys residues. A partially oxidized form of HCox17 with two disulfide bonds and two free cysteines (HCox172S-S) has been proposed to be the functionally competent state in the IMS capable of copper transfer to Sco1 protein partner (11–13). In the latter state, HCox17 is structurally organized in a coiled-coil–helix–coiled-coil–helix (CHCH) domain and binds one copper(I) ion with two consecutive Cys residues at positions C22 and C23 (12). The human Sco1 and Sco2 proteins (HSco1 and HSco2, respectively) contain two essential Cys residues in a fully conserved CXXXC metal-binding motif, arranged in a thioredoxin fold (14–16). These two Cys residues (C169 and C173 in HSco1, C133 and C137 in HSco2), together with a conserved His residue (H260 in HSco1, H224 in HSco2), constitute the copper-binding residues (14, 15). The specific role of these two proteins in the copper transfer to the CuA site is not yet understood and was recently the subject of an extensive scientific debate, in which several different functions, even completely novel and unrelated to the co-chaperone function, have been proposed (14, 17, 18).
Copper binding needs reduced Cys in both donor and accepting proteins. Even in the cytoplasm, although reduced glutathione (GSH) is preponderant with respect to the oxidized form (GSSG), thus providing a reducing environment, some copper-binding sites can require specific proteins to quantitatively reduce all Cys residues, such as glutaredoxin 1 in the case of the Menkes and Wilson proteins (19). No evidence exists for the presence in the IMS of glutathione reductase to maintain GSH/GSSG redox homeostasis (20, 21), thus suggesting that the environment in the IMS can be less reducing than in the cytoplasm, and might depend on its specific regions and cell conditions. Therefore, the question arises about how the accepting copper proteins located in the IMS, e.g., Sco1, Sco2, and CcO subunit II, which bind copper through two Cys residues, are kept reduced. Accordingly, the highly negative reduction potential values of the two metal-binding Cys residues in apoHSco1 and apoHSco2 (14, 15) suggest that a reductant capable of reducing their disulfide bonds is needed for them to be able to bind the copper(I) ion. This feature is even more compelling in Gram-positive and Gram-negative bacteria, where a similar pathway for copper transport and incorporation in CcO, involving Sco proteins (22, 23), occurs outside of the cell or in the periplasmic space, respectively, where Cys residues in Sco and CuA sites can presumably be oxidized by the aerobic environment.
We have already proposed that Cu(I)HSco1 may reduce the disulfide bond in the oxidized apoCuA site of CcO, giving rise to oxidized HSco1 (HSco11S-S hereafter) and copper-bound CuA (14). Here, we show that Cu(I)HCox172S-S, i.e., human Cox17 protein containing two disulfide bridges and one Cu(I) ion bound to two remaining reduced cysteines, is capable of transferring in vitro copper(I) and electrons to HSco11S-S, giving rise to Cu(I)HSco1 and fully oxidized apoHCox173S-S containing three disulfide bonds, thus performing an electron transfer-coupled metallation of HSco11S-S. The same is not true for oxidized HSco2 (HSco21S-S hereafter), although the copper transfer from Cu(I)HCox172S-S to reduced apoHSco2 (apoHSco22SH) occurs similarly to what has already been found for reduced apoHSco1 (apoHSco12SH) (11).
Results
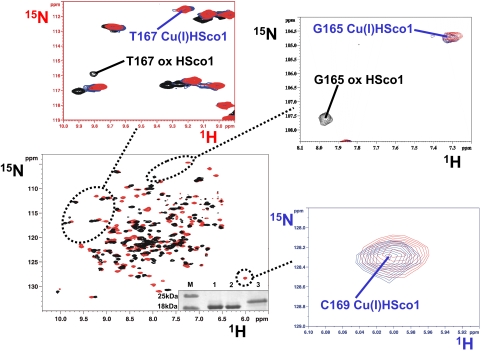
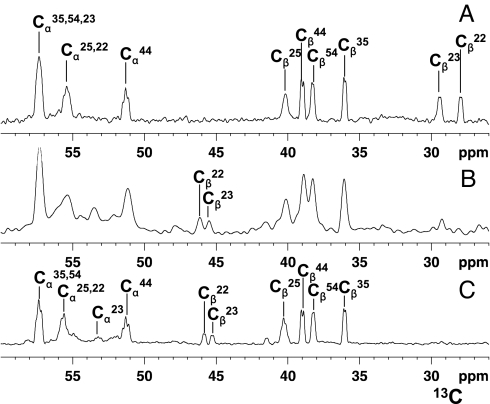
When Cu(I)HCox172S-S is mixed with apoHSco11S-S, i.e., with the two cysteines of the copper-binding site in the oxidized state forming an intramolecular disulfide bond, we observe the simultaneous transfer of copper(I) and of two electrons from Cu(I)HCox172S-S to HSco11S-S, obtaining Cys-reduced Cu(I)HSco1 and apoHCox173S-S. This process was monitored through NMR. The NMR signals of 15N-labeled apoHSco11S-S, titrated with unlabeled Cu(I)HCox172S-S in the absence of reducing agents, indicated the formation of the Cu(I)HSco1 species, as monitored from the appearance of its typical NH resonances in the 1H–15N heteronuclear single quantum coherence (HSQC) spectra (Fig. 1). As an example, the NH cross-peaks of Gly 165 and Thr 167, which are close to the CXXXC metal binding motif and whose 1H,15N chemical shifts drastically change depending on the redox and/or metallation state of HSco1, disappear upon addition of Cu(I)HCox172S-S from the position it has in apoHSco11S-S with the simultaneous appearance of the corresponding cross-peaks, typical of the Cu(I)HSco1 species (Fig. 1). Accordingly, the NH of C169, not detected in apoHSco11S-S, appears in the 1H–15N HSQC spectra of the protein mixture, with the same chemical shift as the Cu(I)HSco1 species (Fig. 1). The changes on HCox172S-S cannot be followed by 1H–15N HSQC spectra because the NH signals of both C22 and C23, involved in copper(I) binding and redox reaction with HSco11S-S, and of the only two other residues (A24 and C25) whose NH chemical shifts are significantly affected by metallation and/or redox state changes (12), broaden beyond detection when Cu(I)HCox172S-S is mixed with apoHSco11S-S [supporting information (SI) Fig. S1]. Broadening of signals, still being detectable, occurs upon addition of apoHSco11S-S also for other NH resonances of HCox172S-S mainly located in the α-helical hairpin (Fig. S1). This behavior originates from exchange processes through a HSco1/Cu(I)1/HCox172S-S transient low-populated intermediate (11). This broadening is detected only for HCox172S-S because the difference in 1H–15N chemical shifts is smaller between Cu(I)HCox172S-S and HCox173S-S with respect to the shift observed between oxidized apoHSco1 and Cu(I)HSco1. The relative difference in 1H–15N chemical shifts makes the latter slow on the NMR time scale and makes the former intermediate. The same behavior was also observed when 15N-labeled Cu(I)HCox172S-S was titrated with unlabeled apoHSco12SH in a reducing environment. Therefore, to analyze the redox state of the cysteines in the Cu(I)HCox172S-S/apoHSco11S-S reaction, we produced a sample with 13C-selectively-labeled cysteines and monitored their redox state in the protein mixture on the basis of the 13C chemical shifts of their Cβ carbons. Indeed, the latter shifts have very specific and completely distinct values whether the sulfur atoms are reduced or oxidized to form a disulfide bond (24). The large 13C chemical shift difference ensures that the equilibrium is slow on the NMR time scale. When the 1D 13C NMR spectra of (13C,2H,15N)Cys-selectively labeled HCox172S-S and (13C,2H,15N)Cys-selectively labeled HCox173S-S are compared, the spectral signatures of the two redox states are clearly evident not only from the Cβ shifts but also from the Cα shifts (Fig. 2). So, when (13C, 2H, 15N) Cys-selectively labeled Cu(I)HCox172S-S is mixed with 15N-labeled apoHSco11S-S in the absence of reducing agents, it is observed that HSco11S-S is reduced and metallated, similarly to what was observed in the previous titration, whereas C22 and C23 of HCox172S-S are oxidized, as shown by their 13C chemical shift values, thus forming HCox173S-S (Fig. 2B).
Fig. 1.
Titration of 15N-labeled HSco11S-S with unlabeled Cu(I)HCox172S-S followed through chemical shift changes in the 1H–15N HSQC spectra. The 1H–15N HSQC spectrum of 15N-labeled HSco11S-S (in black) is overlaid with the 1H–15N HSQC spectrum of a 1:1 15N-labeled HSco11S-S/unlabeled Cu(I)HCox172S-S mixture (in red). In enlarged views selected regions of 1H–15N HSQC spectra are shown in which also the 1H–15N HSQC spectrum of 15N-labeled Cu(I)HSco1 (in blue) is overlaid. The assignment of the NH resonances of G165 and T167 in Cu(I)HSco1 and HSco11S-S forms is reported. NH resonance of C169 is detected only in the copper(I)-bound form. (Inset) Nonreducing SDS/PAGE gel. Lane M, protein marker; lane 1, HSco1; lane 2, HSco11S-S modified with acetamido-4-maleimidylstilbene-2,2-disulfonic acid (AMS); lane 3, AMS-modified HSco12SH.
Fig. 2.
Oxidation state of HCox17 cysteines monitored in the redox reaction between (13C,2H,15N)Cys-selectively labeled Cu(I)HCox172S-S and 15N-labeled HSco11S-S. Chemical shift changes of Cα and Cβ carbons of Cys residues of Cu(I)HCox172S-S were followed by 1D 13C NMR spectra. (A) (13C,2H,15N)Cys-selectively labeled Cu(I)HCox172S-S. (B) 1:1 (13C,2H,15N)Cys-selectively labeled Cu(I)HCox172S-S/15N-labeled HSco11S-S mixture. (C) (13C,2H,15N)Cys-selectively labeled HCox173S-S obtained by air oxidation. The assignment of the Cα and Cβ resonances of the Cys residues of both Cu(I)HCox172S-S and HCox173S-S forms is reported. Cβ signals of the two Cys residues (C22 and C23) of Cu(I)HCox172S-S that are involved in the disulfide exchange reaction drastically reduce their intensity in the protein mixture (B) with the concomitant formation of the corresponding Cβ signals with chemical shifts typical of HCox173S-S.
When unlabeled apoHCox172S-S is mixed with 15N-labeled apoHSco11S-S in the absence of reducing agents, apoHSco12SH is not formed appreciably. The NH resonances typical of apoHSco12SH (i.e., T167 and G165) are indeed not observed in the 1H–15N HSQC spectra of the mixture, but a new set of signals not belonging to either oxidized or reduced apoHSco1 appeared, originating from a species in slow exchange on the chemical shift time scale (Fig. S2). This observations suggests that apoHSco11S-S is interacting with apoHCox172S-S although the redox reaction does not occur appreciably. Addition of copper(I) to the latter apoHCox172S-S/apoHSco11S-S mixture produces the same species obtained by mixing Cu(I)HCox172S-S and apoHSco11S-S, proving that the presence of copper(I) is necessary to promote the disulfide exchange redox reaction (Fig. S2).
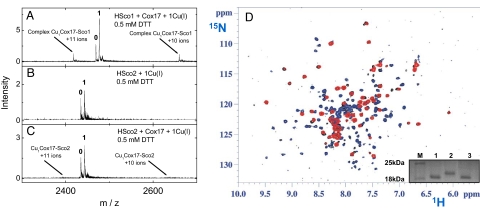
When Cu(I)HCox172S-S is mixed with apoHSco21S-S no reaction occurs, at variance with what is found for apoHSco11S-S, as monitored through 1H–15N HSQC spectra analysis (Fig. 3D and Fig. S1). However, when working in a reducing environment, through addition of DTT at millimolar concentrations, the behavior of the two proteins is similar. Indeed, similarly to what already found for apoHSco12SH (11), Cu(I)HCox172S-S quantitatively transfers the metal ion to apoHSco22SH (Fig. S3). ESI MS experiments performed in the presence of reducing agent (0.5 mM DTT) did not detected any complex between Cu(I)HCox172S-S and HSco2 (Fig. 3C). This finding is at variance with what was observed for the Cu(I)HCox172S-S–HSco1 interaction, in which protein–protein interaction occurs with formation of ≈5% of the complex at micromolar concentrations of the proteins (Fig. 3A). Accordingly, no broadening of NH resonances of HCox172S-S was observed in the mixture of Cu(I)HCox172S-S and apoHSco22SH (Fig. S3), at variance with what was observed in Cu(I)HCox172S-S/HSco1 interactions (Fig. S1), indicating essentially the absence of an equilibrium involving a stable protein–protein complex. Therefore, we suggest that the Cu(I) ion transfer to HSco2 passes through a very low populated complex that is not detected.
Fig. 3.
Electrospray ionization (ESI) MS analysis of Cu(I)HCox172S-S/HSco protein–protein complex formation in the presence of 0.5 mM DTT (Left) and NMR data on Cu(I)HCox172S-S/HSco21S-S mixture in the absence of reducing agents (Right). (A) Mixture of HSco1 (3 μM), HCox172S-S (2 μM), and Cu(I)DTT (2 μM). (B) Mixture of HSco2 (3 μM) and Cu(I)DTT (2 μM). (C) Mixture of HSco2 (3 μM), HCox172S-S (2 μM), and Cu(I)DTT (2 μM). Conditions: 20 mM ammonium acetate, pH 7.5, 0.5 mM DTT. ESI MS spectra were recorded as described in Materials and Methods. Charge state +8 ions are presented for HSco1 and HSco2, and numbers on the peaks denote the metal stoichiometry of the complex. Peaks corresponding to +10 and +11 ions of HCox172S-S/Cu(I)1/HSco1 complexes (A) and positions of theoretical peaks for HCox172S-S/Cu(I)1/HSco2 complexes (C) are indicated with arrows. (D) 1H–15N HSQC spectrum of a 1:1 15N-labeled HSco22S-S/15N-labeled Cu(I)HCox172S-S mixture (in black) is overlaid with 1H–15N HSQC spectra of 15N-labeled HSco21S-S (in blue) and of 15N-labeled Cu(I)HCox172S-S (in red). The NH resonances of the mixture and of the starting materials are well superimposed and no new NH resonances of Cu(I)HSco1 and apoHCox172S-S species are detected, indicating that no reaction occurs. (Inset) Nonreducing SDS/PAGE gel. Lane M, protein marker; lane 1, HSco2; lane 2, AMS-modified HSco22SH; lane 3, AMS-modified HSco21S-S.
Discussion
Recent studies overthrow the previously held view that the presence or absence of disulfide bonds in proteins is determined by the cell compartment and that the simple ratio between reduced and oxidized glutathione in all cases determines the status of intracellular thiol groups (25). Instead, the specific nature of the proteins and their interactions with other proteins determine the redox states of Cys residues. Indeed, glutaredoxin 1 has been proposed to be necessary for completely maintaining the Cys copper(I) ligands of Menkes and Wilson proteins in the reduced state (19), despite the fact that they are in the reducing environment of the cytoplasm. Moreover, a number of proteins have been also found to oxidize Cys in the IMS according to specific pathways (26). Overall, the redox status of thiol groups is in equilibrium with the redox potential of the specific cellular compartment, but apparently this thermodynamic equilibrium involving disulfide formation and electron flow is strongly modulated by protein–protein interactions so that, actually, only some pathways are allowed.
In this frame, we are here suggesting a possible dual functional role for HCox17, which involves the simultaneous reduction of the metal-binding Cys residues of HSco1 and the metal transfer to HSco1. The latter has a thioredoxin fold and was also proposed to have a function not only of copper transfer but also of reducing the Cys residues of the CuA site in CcO (14). Coupling of metal transfer with specific redox chemistry adds an additional level of control into the metal transfer pathway, warranting in particular a high metal specificity of this process for proteins capable of binding metals other than copper with high affinity, as occurs for the Sco1 protein family (14, 27). Such a specificity of HCox172S-S action toward HSco11S-S metallation is highlighted by the fact that redox reaction does not occur with HSco21S-S. HCox17 can therefore work to specifically metallate and reduce HSco11S-S with respect to HSco21S-S, being able in this way to select between two proteins with similar copper(I)-binding affinity.§ Because the redox potentials of apoHSco11S-S and apoHSco21S-S are similar (11, 15), the different behavior of the two proteins toward redox chemistry has to rely on kinetic grounds, which might arise from the occurrence or absence of specific protein–protein interactions. Previously we demonstrated by ESI MS that Cu(I)HCox172S-S and HSco12SH form a specific metal-bridged HSco1/Cu(I)1/HCox172S-S complex, which might serve also as transient metal transfer complex in vivo (11). Current ESI MS results confirm the formation of a specific low fraction (≈5%) metal-bridged HSco1/Cu(I)1/HCox172S-S complex at micromolar concentrations of proteins. However, in similar conditions, no adduct was detected between Cu(I)HCox172S-S and HSco22SH. The specific protein–protein interactions between HSco1 and HCox17 might therefore be crucial for electron transfer from Cu(I)HCox172S-S to apoHSco11S-S, thus establishing the molecular grounds of the selectivity of redox chemistry observed between HCox172S-S and HSco11S-S with respect to HCox172S-S and HSco21S-S. The protein–protein interaction can be indeed crucial to position the disulfide bond of HSco1 in the correct orientation facilitating electron transfer reaction.
This view is in agreement with previous findings showing that the metallation of HSco1, but not of HSco2, when expressed in the yeast cytoplasm, depends on the coexpression of HCox17 (28), suggesting that HCox17 is the specific protein partner of HSco1. Therefore, the nonoverlapping functions of HSco1 and HSco2 proteins in mitochondrial copper delivery (7) can rely on their different redox behavior when interacting with Cu(I)HCox172S-S but not on their copper-binding capabilities, which are indeed similar. This finding could be important to establish the molecular basis for the distinct clinical presentation of patients with mutations in Sco2 with respect to that of Sco1 patients (8). The failure of electron and copper transfer processes from HCox172S-S to HSco2 also suggests that an additional partner of HSco2 could be necessary to reduce its disulfide bond to allow the copper(I) incorporation into the CuA site. The recently observed different backbone dynamic properties of apoHSco2 with respect to apoHSco1 (15) can indeed play an important role in selecting different protein partners in the molecular recognition process.
HCox173S-S is quickly reduced in vitro to HCox172S-S by millimolar concentrations of GSH (13). It is reasonable then to assume that, after each redox metal transfer cycle, the fully oxidized HCox173S-S could be reduced by GSH in the IMS and the protein be recycled. Because the reduction potential of the redox couple apoHSco11S-S/apoHSco10S-S is more negative (−280 mV) (11) than that of apoHCox173S-S/apoHCox172S-S (−198 mV) (13), the thermodynamic equilibrium disfavors the transfer of electrons to apoHSco11S-S. Indeed, when apoHCox172S-S was mixed with apoHSco11S-S, no disulfide exchange reaction was observed. Therefore, the presence of copper(I) is necessary to drive the electrons toward the reduction of apoHSco11S-S and the driving force of electron flow from HCox172S-S to HSco11S-S is determined by the higher thermodynamic stability of Cu(I)HSco1 with respect to that of Cu(I)HCox172S-S (11).
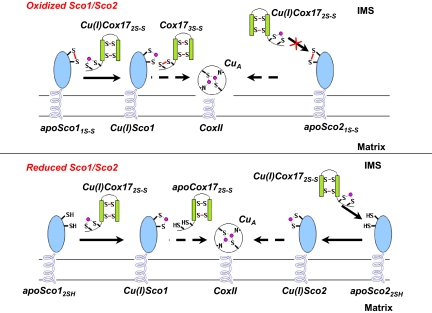
The in vitro observation of the simultaneous electron and copper transfer reaction between Cu(I)HCox172S-S and apoHSco11S-S suggests that the same reaction can occur also in vivo when needed: copper(I) eventually transferred to the CuA site, with HSco1 acting as a thioredoxin (14). Living organisms could have, therefore, developed an efficient and rapid mechanism of metal incorporation capable of handling the complex redox scenario of IMS, enabling transfer of copper(I) to the CuA site both when Sco1 protein is in an oxidized and when it is in a reduced state. Our intriguing hypothesis involves HCox172S-S as the origin of an electron cascade for the reduction of the Cys residues in the CuA site, through HSco1 (Fig. 4).
Fig. 4.
Proposed mechanism of copper transfer from HCox172S-S to the subunit II of CcO, through the assistance of HSco1 and HSco2. This model implies that HSco1, independently of the redox state of its Cys ligands in the IMS, may accept copper(I) from the mitochondrial chaperone Cu(I)HCox172S-S to form Cu(I)HSco1 whereas HSco2 can accept copper(I) from Cu(I)HCox172S-S only once its Cys ligands in the IMS are in a reduced state. HCox173S-S, produced by the redox HSco1/HCox172S-S reaction, can be quickly reduced to HCox172S-S by GSH, thus the latter protein being recycled for the following metal transfer.
In conclusion, from this work we propose that copper trafficking in the IMS is a complex process requiring multiple steps that need to deal with various redox states of the involved proteins, in a system-dependent manner.
Materials and Methods
Protein Expression and Purification Procedures.
Unlabeled and uniformly 15N-labeled HSco1, HSco2, and HCox172S-S proteins were expressed and purified from Escherichia coli by following already reported protocols (12, 14, 15). Aerobic oxidation of HSco1 and HSco2 were followed through nonreducing SDS/PAGE gels after AMS sample modification. Metallation of HCox172S-S was performed by following a previously reported protocol (12). To produce (13C,2H,15N)Cys-selectively labeled HCox172S-S, the Cox17-pETG-30A expression vector was transformed in a suitable cysteine-auxotrophic strain, BL21(DE3)cysE (29, 30), and the cells were grown in a minimal medium supplemented with 50 mg/liter of l-(U-13C3,U-2H3,15N)cysteine (Cambridge Isotope Laboratories; each isotope at 98% replacement), 100 mg/liter each other unlabeled amino acid, 3 g/liter glucose, 1 g/liter (NH4)2SO4. The growth of BL21(DE3)cysE/Cox17 cells and overexpression of (13C,2H,15N)Cys-selectively labeled HCox172S-S were carried out in a 2.5-liter Tunair shaking flask (IBI-Shelton Scientific) with a working solution of 0.5 liter. The culture was grown at 37°C up to OD600 = 0.8 and then the protein expression was induced by addition 0.7 mM IPTG at 25°C for 16 h. Purification and metallation of (13C,2H,15N)Cys-selectively labeled HCox172S-S were performed by following the protocol in ref. 12.
The numbering of HCox17 sequence follows the isolated functional mammalian Cox17 sequences (31, 32), in which the first Met is processed away posttranslationally. Therefore, the HCox17 sequence numbering starts with Pro-1.
NMR Titration of the Two Proteins.
Titrations of Cu(I)HCox172S-S with apoHSco11S-S were performed with NMR spectroscopy, following the 1H–15N and 13C chemical shift changes upon the addition of increasing amounts of the titrating protein. Two-dimensional 1H–15N HSQC and/or 1D 2H-decoupled 13C experiments were collected at 298 K during the titrations on Avance 900, 800, and 500 Bruker spectrometers operating at a proton nominal frequency of 900.13 MHz, 800.13 MHz, and 500.13 MHz, respectively. Aliquots were added in a Coy chamber under nitrogen atmosphere at 298 K to deliver unlabeled or labeled proteins to the labeled samples in NMR tubes. The titrations were performed in the absence of reducing agents, adding unlabeled Cu(I)HCox172S-S or 15N-labeled apoHSco11S-S to 15N-labeled apoHSco11S-S or (13C,2H,15N)Cys-selectively labeled Cu(I)HCox172S-S, respectively, and unlabeled apoHSco11S-S to 15N-labeled Cu(I)HCox172S-S. Protein mixture concentrations range between 0.1 and 0.4 mM. Similarly, HSco2/HCox17 interaction has been followed by NMR both in the presence (1 mM DTT) and in the absence of reducing agents. In the first case, 15N-labeled apoHSco22SH or 15N-labeled Cu(I)HCox172S-S was titrated with unlabeled Cu(I)HCox172S-S or unlabeled apoHSco22SH, respectively. In the second case, 15N-labeled apoHSco21S-S was titrated with 15N-labeled Cu(I)HCox172S-S and 15N-labeled Cu(I)HCox172S-S, with unlabled HSco21S-S. For all titrations, protein/protein ratio ranges from 0 to 2 equivalents.
ESI MS Studies.
Cu(I)HCox172S-S was prepared by addition of 1 eq of Cu(I)DTT complex to HCox172S-S in 20 mM ammonium acetate, pH 7.5, containing 0.5 mM DTT. Cu(I)·DTT complex was prepared by dissolving of 150 μM Cu(II)acetate in argon-saturated 20 mM ammonium acetate, pH 7.5, containing 1 mM DTT. The Cu(I)HCox172S-S sample obtained was subsequently mixed with the sample of HSco1 or HSco2 protein in 20 mM ammonium acetate, pH 7.5, containing 0.5 mM DTT. The obtained mixture containing 2 μM Cu(I)HCox172S-S and 3 μM HSco1 or HSco2 was incubated for an additional 1 min at 298 K and injected by a syringe pump at 5 μl/min into the electrospray ion source of a QSTAR Elite ESI-Q-TOF MS instrument (Applied Biosystems). ESI MS spectra were recorded in the region from 500 to 3,000 Da at the following instrument parameters: ion spray voltage 5,500 V; source gas 30 liters/min; curtain gas 20 liters/min; declustering potential 60 V; focusing potential 320 V; detector voltage 2,300 V. ESI MS spectra were analyzed by the program Analyst (Applied Biosystems). Fractional content of the HCox172S-S/Cu(I)1/HSco1 complex was calculated from peak intensities, taking into account all charge states of HSco1 (+9 and +8) and of the complex (+10 and +11) in the ESI MS spectra, assuming that ionization efficiency for all forms of HSco1 as well as of the HCox172S-S/Cu(I)1/HSco1 complex is similar.
Supplementary Material
Acknowledgments.
We thank Marie-Paule Strub for providing us the cysteine-auxotrophic strain BL21(DE3)cysE. This work was supported by the European Commission (“European Network of research Infrastructures for providing Access and Technological Advancements in bio-NMR,” Contract 026145; “From Receptor to Gene: structures of complexes from signaling pathways linking immunology, neurobiology and cancer,” SPINE2-COMPLEXES Contract LSHG-CT-2006-031220). The Italian Ministero dell'Universitá e della Ricerca Scientifica e Technologica COFIN03 and MIUR–FIRB (Ministero dell'Università e della Ricerca–Fondo per gli Investimenti della Ricerca di Base, Protocollo MIUR-RBLA032ZM7), and the Estonian Science Foundation (Project 7191) are also acknowledged for financing.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800019105/DCSupplemental.
When, to a 1:1 mixture of 15N-labeled apoHSco1 and 15N-labeled apoHSco2, 1 eq of a copper(I) acetonitrile complex was added, formation of the copper form for the two proteins was observed in similar ratios, thus indicating similar cooper(I)-binding affinity (Fig. S4); the copper forms are in a slow exchange on the chemical shift time scale with the apo forms.
References
- 1.Ramirez BE, Malmström BG, Winkler JR, Gray HB. The currents of life: The terminal electron-transfer complex of respiration. Proc Natl Acad Sci USA. 1995;92:11949–11951. doi: 10.1073/pnas.92.26.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finney LA, O'Halloran TV. Transition metal speciation in the cell: Insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 3.Tsukihara T, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 Å. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 4.Tsukihara T, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 5.Carr HS, Winge DR. Assembly of cytochrome c oxidase within the mitochondrion. Acc Chem Res. 2003;36:309–316. doi: 10.1021/ar0200807. [DOI] [PubMed] [Google Scholar]
- 6.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J Biol Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 7.Leary SC, et al. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 8.Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet. 2001;106:46–52. doi: 10.1002/ajmg.1378. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, et al. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome c oxidase and embryonic development. Mol Cell Biol. 2002;22:7614–7621. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glerum DM, Shtanko A, Tzagoloff A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J Biol Chem. 1996;271:14504–14509. doi: 10.1074/jbc.271.24.14504. [DOI] [PubMed] [Google Scholar]
- 11.Banci L, et al. Human Sco1 functional studies and pathological implications of the P174L mutant. Proc Natl Acad Sci USA. 2007;104:15–20. doi: 10.1073/pnas.0606189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banci L, et al. A structural-dynamical characterization of human Cox17. J Biol Chem 283. 2008;283:7912–7920. doi: 10.1074/jbc.M708016200. [DOI] [PubMed] [Google Scholar]
- 13.Voronova A, et al. Oxidative switches in functioning of mammalian copper chaperone Cox17. Biochem J. 2007;408:139–148. doi: 10.1042/BJ20070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banci L, et al. A hint for the function of human Sco1 from different structures. Proc Natl Acad Sci USA. 2006;103:8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banci L, et al. A structural characterization of human Sco2. Structure. 2007;15:1132–1140. doi: 10.1016/j.str.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Williams JC, et al. Crystal structure of human SCO1: Implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J Biol Chem. 2005;280:15202–15211. doi: 10.1074/jbc.M410705200. [DOI] [PubMed] [Google Scholar]
- 17.Leary SC, et al. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 19.Lim CM, Cater MA, Mercer JF, La Fontaine S. Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases. Biochem Biophys Res Commun. 2006;348:428–436. doi: 10.1016/j.bbrc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 20.Koehler CM, Beverly KN, Leverich EP. Redox pathways of the mitochondrion. Antioxid Redox Signal. 2006;8:813–822. doi: 10.1089/ars.2006.8.813. [DOI] [PubMed] [Google Scholar]
- 21.Khalimonchuk O, Winge DR. Function and redox state of mitochondrial localized cysteine-rich proteins important in the assembly of cytochrome c oxidase. Biochim Biophys Acta. 2008;1783:618–628. doi: 10.1016/j.bbamcr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banci L, et al. A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc Natl Acad Sci USA. 2005;102:3994–3999. doi: 10.1073/pnas.0406150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnesano F, Banci L, Bertini I, Martinelli M. Ortholog search of proteins involved in copper delivery to cytochrome c oxidase and functional analysis of paralogs and gene neighbors by genomic context. J Proteome Res. 2005;4:63–70. doi: 10.1021/pr049862f. [DOI] [PubMed] [Google Scholar]
- 24.Sharma D, Rajarathnam K. 13C NMR chemical shifts can predict disulfide bond formation. J Biomol NMR. 2000;18:165–171. doi: 10.1023/a:1008398416292. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann JM, Köhl R. Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. J Cell Biol. 2007;176:559–563. doi: 10.1083/jcb.200611060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesecke N, et al. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005;121:1059–1069. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Andruzzi L, Nakano M, Nilges MJ, Blackburn NJ. Spectroscopic studies of metal binding and metal selectivity in Bacillus subtilis BSco, a homologue of the yeast mitochondrial protein Sco1p. J Am Chem Soc. 2005;127:16548–16558. doi: 10.1021/ja0529539. [DOI] [PubMed] [Google Scholar]
- 28.Horng YC, et al. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 29.Müller S, et al. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: Biosynthesis and characterization of Se2-thioredoxin. Biochemistry. 1994;33:3404–3412. doi: 10.1021/bi00177a034. [DOI] [PubMed] [Google Scholar]
- 30.Strub M-P, et al. Selenomethionine and selenocysteine double labeling strategy for crystallographic phasing. Structure. 2003;11:1359–1367. doi: 10.1016/j.str.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZW, et al. Characterization of dopuin, a polypeptide with special residue distributions. Eur J Biochem. 1997;249:518–522. doi: 10.1111/j.1432-1033.1997.t01-2-00518.x. [DOI] [PubMed] [Google Scholar]
- 32.Takenouchi T, Fujimoto M, Shimamoto A, Munekata E. Isolation and characterization of Cox17p from porcine heart by determining its survival-promoting activity in NIH3T3 cells. Biochim Biophys Acta. 1999;1472:498–508. doi: 10.1016/s0304-4165(99)00158-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.