Abstract
We recently demonstrated early metabolic alterations in the dystrophin-deficient mdx heart that precede overt cardiomyopathy and may represent an early “subclinical” signature of a defective nitric oxide (NO)/cGMP pathway. In this study, we used genetic and pharmacological approaches to test the hypothesis that enhancing cGMP, downstream of NO formation, improves the contractile function, energy metabolism, and sarcolemmal integrity of the mdx heart. We first generated mdx mice overexpressing, in a cardiomyocyte-specific manner, guanylyl cyclase (GC) (mdx/GC+/0). When perfused ex vivo in the working mode, 12- and 20-week-old hearts maintained their contractile performance, as opposed to the severe deterioration observed in age-matched mdx hearts, which also displayed two to three times more lactate dehydrogenase release than mdx/GC+/0. At the metabolic level, mdx/GC+/0 displayed a pattern of substrate selection for energy production that was similar to that of their mdx counterparts, but levels of citric acid cycle intermediates were significantly higher (36 ± 8%), suggesting improved mitochondrial function. Finally, the ability of dystrophin-deficient hearts to resist sarcolemmal damage induced in vivo by increasing the cardiac workload acutely with isoproterenol was enhanced by the presence of the transgene and even more so by inhibiting cGMP breakdown using the phosphodiesterase inhibitor sildenafil (44.4 ± 1.0% reduction in cardiomyocyte damage). Overall, these findings demonstrate that enhancing cGMP signaling, specifically downstream and independent of NO formation, in the dystrophin-deficient heart improves contractile performance, myocardial metabolic status, and sarcolemmal integrity and thus constitutes a potential clinical avenue for the treatment of the dystrophin-related cardiomyopathies.
Keywords: cardiomyopathy, isotopes, perfusion, energy metabolism, nitric oxide
Dystrophin is a large subsarcolemmal protein, which exists as part of a multimolecular network (the dystrophin–glycoprotein complex) that spans the plasma membrane and links the intracellular cytoskeleton to the extracellular matrix (1, 2). Abnormalities of dystrophin are the proximate cause of the skeletal and cardiac disease associated with Duchenne/Becker muscular dystrophies (DMD/BMD) and, of potentially broader clinical relevance, have recently been linked to several acquired forms of cardiomyopathy and proposed to be a common pathway for contractile dysfunction in the failing myocardium (3–5). Despite enormous progress in identifying the genetic and biochemical abnormalities associated with dystrophin deficiency, particularly in skeletal muscle, the functional role of dystrophin in the heart needs to be better understood for the design of more effective treatment strategies for DMD and other dystrophin-related cardiomyopathies.
The most widely used animal model of DMD, the mdx mouse, lacks dystrophin due to a spontaneous nonsense mutation within exon 23 of the murine dystrophin gene (6). We have shown that even at 8–10 weeks of age, when no cardiac pathology is evident by histology or echocardiography, mdx mouse hearts are abnormally vulnerable to mechanical stress and workload-induced damage (7). More recently, we simultaneously characterized the metabolic and functional profile of these dystrophin-deficient hearts using ex vivo perfusion with carbon 13 (13C)-labeled substrates. We demonstrated precocious metabolic and functional abnormalities, which preceded the development of overt cardiomyopathy (8), and suggested this may represent an early “subclinical” signature of defective nitric oxide (NO) and/or cGMP signaling.
NO mediates a large number of physiological and pathophysiological effects via its cellular effector cGMP and cGMP-independent mechanisms (9). In dystrophin-deficient skeletal muscle, neuronal NO synthase (nNOS, one of the enzymes responsible for NO production) is displaced away from its normal subsarcolemmal location to the cytoplasm, where its mislocalization and decreased activity level are thought to contribute to muscle pathology (10–13). In the dystrophic heart, a significant down-regulation of nNOS activity has also been reported to occur (14). Conversely, overexpression of an nNOS transgene in the mdx mouse heart was found to mitigate the inflammation, fibrosis, and electrocardiographic abnormalities that develop in older mdx mice (15). However, it remains to be determined whether these beneficial effects on dystrophic cardiomyopathy can be achieved solely through enhancement of cGMP-mediated signaling, without the requirement for increased NO production. This is particularly important because drugs that inhibit cGMP breakdown, such as the phosphodiesterase 5 (PDE5) inhibitor, sildenafil, are already available for clinical use and have been shown to blunt cardiac hypertrophy and improve myocardial function in mice subjected to pressure overload (16).
Accordingly, in this study, we wished to test the hypothesis that early activation of the cGMP pathway, specifically downstream and independent of NO formation, is able to confer beneficial effects on the mdx mouse heart. We first generated mdx mice with cardiomyocyte-specific overexpression of a constitutively active form of guanylyl cyclase (mdx/GC+/0). In wild-type animals, the presence of this transgene, which increases cytoplasmic production of cGMP, inhibits isoproterenol- and pressure overload-induced hypertrophy (17). Using validated experimental paradigms (7, 8), we tested the ability of the mdx/GC+/0 heart to withstand: (i) ex vivo perfusion in the working mode, with concomitant evaluation of myocardial contractility and metabolic profile parameters at different points in the natural evolution of the dystrophic cardiomyopathy; and (ii) an acute increase in the cardiac workload induced in vivo by β-adrenergic stimulation. Further to our findings of improved contractility, energy metabolism, and cellular integrity in mdx/GC+/0 hearts, we then treated young (6-week-old) mdx mice with the PDE5 inhibitor sildenafil and found similar beneficial effects. Taken together, our findings point to defective cGMP signaling as being an important component of disease pathogenesis in the dystrophin-deficient heart and suggest the basis for a previously undescribed therapeutic approach to prevent or delay the onset of dystrophin-related cardiomyopathies.
Results
Transgenic Approach: Cardiomyocyte-Specific Overexpression of Constitutively Active GC Improves Sarcolemmal Integrity, Myocardial Contractile Function, and Mitochondrial Metabolism.
To test our primary hypothesis that early activation of the cGMP pathway can confer beneficial effects on the dystrophin-deficient heart, we first analyzed mdx/GC+/0 mice and their nontransgenic mdx counterparts using our isolated working mouse heart model perfused with a mixture of substrates mimicking the in vivo milieu. We have reported that even at 12 weeks of age, when no significant echocardiographic or histologic abnormalities are present, mdx hearts perfused ex vivo in the working mode suffer greater cardiomyocyte damage [as indicated by excessive lactate dehydrogenase (LDH) release] and display a reduced ability to sustain normal contractile function, particularly when supplied with glucose as the only substrate (7, 8). In this study, comparisons were made between mdx and mdx/GC+/0 hearts at two different ages, 12 and 20 weeks, to determine whether differences related to expression of the transgene become more pronounced as the disease progresses with advancing age.
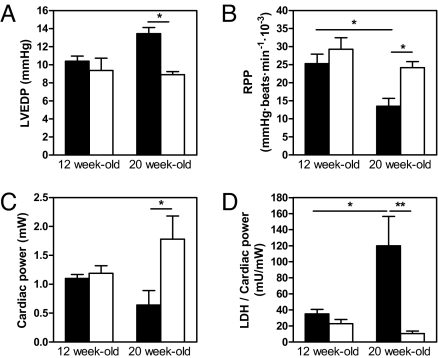
At 12 weeks of age, the mdx and mdx/GC+/0 groups displayed similar values for multiple contractile function parameters when placed in the working heart mode. However, there was a very clear deterioration of myocardial function observed with advancing age in mdx hearts, whereas the transgenic mdx/GC+/0 mice demonstrated a remarkable ability to maintain a stable level of contractile performance between 12 and 20 weeks. As indicated in Fig. 1 and supporting information (SI) Table S1, this resulted in significant differences between the mdx and mdx/GC+/0 groups for left ventricular end-diastolic pressure, rate-pressure product, cardiac output, and cardiac power at 20 weeks of age. Interestingly, the indices of contractility and relaxation (+dP/dt and −dP/dt) and the rate-pressure product were significantly decreased with age in the mdx group but were maintained in the mdx/GC+/0 group (Table S1 and Fig. 1B). We confirmed that constitutive overexpression of guanylate cyclase in the mdx heart was able to significantly up-regulate cGMP levels (1.9 ± 0.3 vs. 1.1 ± 0.1 fmol/100 mg of heart wet weight, in perfused mdx/GC+/0 animals vs. their mdx counterpart, respectively; P < 0.05). Furthermore, in both age groups examined, the mdx/GC+/0 hearts exhibited lower levels of LDH release (Table S1). In fact, when expressed relative to cardiac power, LDH release tended to decrease with age in mdx/GC+/0 hearts (P = 0.09), whereas it increased significantly (P < 0.05) in nontransgenic mdx hearts (Fig. 1D).
Fig. 1.
Functional and physiological parameters of 12- and 20-week-old isolated working mdx and mdx/GC+/0 mouse hearts. Data are means ± SEM of mdx (solid bars) and mdx/GC+/0 (open bars) heart perfusion experiments (n = 4 mice per group). Values shown represent averages for the 20- to 30-min perfusion period. *, P < 0.05; **, P < 0.001 mdx vs. mdx/GC+/0 mouse hearts. LVEDP, left ventricular end-diastolic pressure; RPP, rate-pressure product.
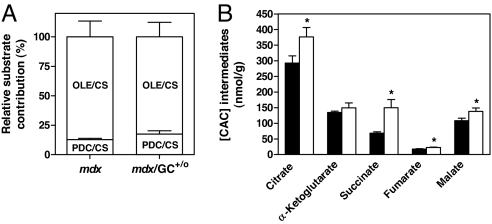
In the aforementioned perfusion studies, we simultaneously evaluated the metabolic profile of the hearts using substrates labeled with stable isotopes (13C). This approach allows for detailed and simultaneous measurements of the dynamics of cardiac energy substrate metabolism, information not accessible from static measurements of mRNA or protein expression. We showed that the mdx heart displays a metabolic shift toward a preferential use of carbohydrates for energy production and a significant decrease in mitochondrial citric acid cycle (CAC) pool size (8). [U-13C3]pyruvate and [1-13C18]oleate were used to evaluate the contribution of carbohydrates to acetyl-CoA production and exogenous fatty acids to β-oxidation, respectively. As shown in Fig. 2, at 12 weeks of age mdx/GC+/0 hearts displayed a similar pattern of substrate selection for energy production as nontransgenic mdx (Fig. 2A). However, the mdx/GC+/0 hearts maintained significantly greater levels CAC intermediates under these conditions (Fig. 2B), indicating partial reversal of the previously demonstrated reduction of the CAC pool in mdx hearts (8). It is noteworthy that we were unable to do the same metabolic analysis at 20 weeks of age, because these older mdx hearts were incapable of sustaining contractile function for the time required to assess metabolic fluxes.
Fig. 2.
Flux parameters relevant to citrate synthesis and mitochondrial citric acid cycle (CAC) intermediates in isolated working mdx and mdx/GC+/0 mouse hearts. Data are means ± SEM of perfusion experiments performed in isolated working mdx (solid bars) and mdx/GC+/0 (open bars) mouse hearts (n = 4 per group). Procedures for the determination of (A) the contribution of carbohydrates (lactate, pyruvate, and glucose) and exogenous fatty acids (oleate) to acetyl-CoA formation for citrate synthesis (CS) via pyruvate decarboxylation (PDC) and β-oxidation (OLE), respectively referred to as PDC/CS and OLE/CS and (B) tissue levels of CAC intermediates are described in Materials and Methods. *, P < 0.05 mdx vs. mdx/GC+/0 mouse hearts.
To ascertain the effects of the transgene on cardiomyocyte sarcolemmal integrity in vivo, an acute increase in cardiac workload was induced by isoproterenol infusion. We have shown that hearts from 8- to 10-week-old mdx mice exhibit an abnormally increased susceptibility to sarcolemmal damage upon exposure to this form of acute cardiac stress (7). Although isoproterenol infusion led to a transient and equivalent increase in cardiac frequency (peaking at ≈2.5 times the baseline values) in both nontransgenic mdx and mdx/GC+/0 groups, mdx/GC+/0 hearts were able to maintain this elevated heart rate over a significantly longer period (Fig. 3A). Moreover, the level of cardiomyocyte sarcolemmal injury (as determined by Evans blue dye uptake) was decreased compared to nontransgenic mdx mice (see Fig. 3B), although this difference did not reach statistical significance (P = 0.1).
Fig. 3.
Heart rate responses and quantitation of in vivo cardiomyocyte sarcolemmal injury induced by an acute increase in cardiac mechanical stress after isoproterenol infusion in mdx and mdx/GC+/0 mice. Values are means ± SEM of four to six experiments in mdx (solid bars) or mdx/GC+/0 (open bars) mouse hearts. Heart rate was continuously monitored by a single-lead electrocardiogram (A), and cardiomyocyte sarcolemmal injury was evaluated by using the Evans blue vital dye (B). ***, P < 0.001 for mdx vs. mdx/GC+/0 mouse hearts.
Pharmacological Approach: Sildenafil Treatment of Dystrophin-Deficient Mice Reduces Early Signs of Cardiomyopathic Remodeling and Protects Against Workload-Induced Cardiomyocyte Injury.
To evaluate the therapeutic potential of a pharmacological approach, we treated mdx mice with the PDE5 inhibitor, sildenafil, which is known to enhance cGMP signaling by inhibiting its breakdown (16). The treatment was given daily from 6 to 12 weeks of age. Although mdx/GC+/0 did not differ significantly from nontransgenic mdx in their myocardial contractile performance at 12 weeks of age when studied ex vivo, we wished to exclude any possible superimposed effects of sildenafil on cardiac function related to its ability to act as a peripheral vasodilator in vivo. Therefore, the mice were anesthetized and examined in vivo by using echocardiography and direct intravascular pressure monitoring. None of the echocardiographic or other hemodynamic parameters, including blood pressure, were altered by sildenafil treatment (Table S2). Despite the lack of changes in myocardial contractility in vivo at 12 weeks of age, we have reported that the atrial natriuretic factor (anf) gene expression, a precocious marker of cardiomyopathic remodeling, is significantly increased in the mdx heart even at this early point in the disease process (8). In this study, we found that, compared with the placebo group, mdx mice treated with sildenafil had significantly reduced cardiac mRNA levels of anf (Fig. 4A), which suggests that the drug prevents the development of cardiomyopathic remodeling at early stages of the disease. In addition, a significant down-regulation of the α1 subunit of soluble guanylyl cyclase (sgcα1) gene expression was also observed in sildenafil- as compared with placebo-treated mdx hearts (Fig. 4B), a finding consistent with enhanced cGMP-mediated signaling in the group receiving the active compound (18). The latter notion was further supported by measurements of myocardial cGMP levels. In fact, because previous studies have shown that plasma cGMP levels in mice reach their peak within 1 h after sildenafil administration by the oral route (19), we measured cGMP levels in mdx hearts at 45 and 90 min after i.p. administration of sildenafil. In keeping with this previous pharmacokinetic study, sildenafil administration to mdx mice induced an increase in cGMP levels in the heart at 45 min (3.2 ± 0.6 vs. 1.7 ± 0.4 fmol/100 mg of heart wet weight, in sildenafil- vs. placebo-treated mdx animals, respectively, P = 0.09), with a subsequent return to baseline values by 90 min posttreatment (1.9 ± 0.8 vs. 2.1 ± 0.5 fmol/100 mg of heart wet weight, not significant).
Fig. 4.
Changes in gene expression levels for atrial natriuretic factor (anf) and the α1 subunit of soluble guanylyl cyclase (sgcα1) in placebo vs. sildenafil-treated mdx mice. Data are means ± SEM of eight freeze–clamped placebo (solid bars) and sildenafil-treated (open bars) mdx mouse hearts. mRNA levels were normalized to total myocardial RNA. Statistical analyses were performed on raw data. *, P < 0.05 for placebo vs. sildenafil-treated mdx mouse hearts.
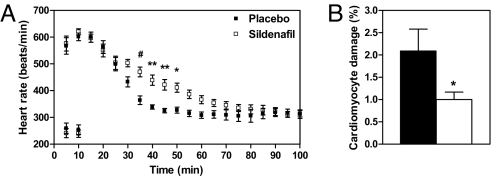
We then assessed whether sildenafil could improve the resistance of mdx hearts to cardiomyocyte damage induced by isoproterenol administration in vivo. As was the case in mdx/GC+/0 hearts, sildenafil-treated hearts were able to sustain their elevated heart rate response for a significantly longer period (Fig. 5A), while showing at the same time a significantly lower level of cardiomyocyte sarcolemmal injury (44.4 ± 1.0% decrease; Fig. 5B). Finally, it should be noted that the protective effects reported in this study cannot be attributed to increased expression of the dystrophin homologue utrophin, because neither immunohistochemistry nor Western blot analysis revealed any evidence of utrophin up-regulation within mdx/GC+/0 or sildenafil-treated hearts (data not shown).
Fig. 5.
Heart rate responses and in vivo cardiomyocyte sarcolemmal injury induced by an acute increase in cardiac mechanical stress in placebo vs. sildenafil-treated mdx mice. Values are means ± SEM of four to six experiment in placebo (solid bars) or sildenafil-treated (open bars) mdx mouse hearts, as described in Fig. 3. *, P < 0.05; **, P < 0.01, #, P < 0.001 for placebo vs. sildenafil-treated mdx mouse hearts.
Discussion
In this study, we have used complementary genetic and pharmacological approaches to demonstrate that enhancing cGMP signaling in dystrophin-deficient hearts: (i) protects cardiomyocytes against mechanical workload-induced sarcolemmal damage; (ii) preserves mitochondrial metabolic status; and, most importantly, (iii) prevents the deterioration of myocardial contractile performance usually observed with advancing age in mdx hearts. Our findings are consistent with a previous study by Wehling-Henricks et al. (15), which showed beneficial effects of overexpressing an nNOS transgene in the mdx heart. However, the latter study did not establish whether the salutary effects of nNOS overexpression were mediated by NO-induced activation of soluble GC or by cGMP-independent mechanisms. Here, we show that enhancing cGMP signaling per se protects the dystrophin-deficient heart. Moreover, such cardioprotection does not depend on peripheral systemic effects (as could occur with sildenafil) but is also achieved when cGMP is up-regulated in a cardiomyocyte-specific manner. Therefore, our data suggest that defective cGMP signaling within cardiac muscle cells may play a fundamental role in the pathogenesis of the cardiomyopathy associated with dystrophin deficiency. In addition, our study raises the possibility that a new therapeutic approach based on cGMP up-regulation with PDE5 inhibitors could be an effective treatment for this condition.
In both skeletal and cardiac muscle, it has been proposed that dystrophin participates in signaling functions and acts to mechanically reinforce the sarcolemma, thereby helping to protect muscle cells against workload-induced membrane damage (7, 20). Consistent with this notion, it was reported by Danialou et al. (7) that mdx hearts are more vulnerable to sarcolemmal damage induced by aortic banding or β-adrenergic stimulation, both of which substantially augment the mechanical workload placed on the heart. Using a similar experimental paradigm, we report in this study that cardiomyocyte-specific overexpression of GC and sildenafil treatment in mdx mice both decreased the level of cardiomyocyte injury and simultaneously improved the ability of mdx mice to sustain an increased heart rate during β-adrenergic stimulation in vivo. It is noteworthy that such differences were observed at the young age of 12 weeks, i.e., at a time where there are no echocardiographic signs of cardiomyopathy. At 20 weeks of age, differences in susceptibility to cardiomyocyte injury between mdx/GC+/0 and nontransgenic mdx hearts became even more evident in the working heart perfused ex vivo, a procedure that may itself be considered a mild form of “cardiac stress.” In fact, mdx/GC+/0 hearts released significantly less LDH despite maintaining a substantially greater workload, as reflected by an ≈3-fold higher value for cardiac power. Even more striking, enhancing cGMP signaling in cardiomyocytes was able to mitigate cardiac disease progression, as evidenced by the preservation of myocardial contractility between 12 and 20 weeks of age in mdx/GC+/0 hearts, in marked contrast to the substantial loss of function observed in mdx hearts during the same time frame.
We have shown that the ex vivo perfusion environment reveals cardiomyopathic changes in mdx hearts, which are otherwise masked by homeostatic compensatory mechanisms present within the intact animal. Using this approach combined with stable isotope methodology, we documented a metabolic shift in substrate utilization from fatty acid to carbohydrate oxidation in mdx compared with dystrophin-expressing control hearts (8). In the present study, mdx/GC+/0 mice displayed improved myocardial function and resistance of cardiomyocytes to workload-induced injury compared with mdx mice. However, both mdx and mdx/GC+/0 hearts demonstrated a similar substrate selection for energy production, which suggests that the metabolic shift reported in mdx hearts may be an adaptive response rather than a deleterious consequence of cardiac dysfunction. Potential benefits associated with this change in substrate utilization could include: (i) a greater ATP-to-oxygen ratio associated with glucose oxidation; (ii) a more efficient matching between cytosolic glycolysis and mitochondrial oxidation, thereby decreasing the production of protons, a factor that can promote detrimental calcium overload; and (iii) improved ion pump function linked to glycolytic flux (for review, see ref. 21).
However, beyond the shift in substrate selection, it is important to note that the CAC pool size was significantly increased in perfused mdx/GC+/0 as compared with mdx hearts, suggesting an improved mitochondrial metabolic status in the former group. Such a change would be expected to enable the mdx heart to enhance CAC flux and hence mitochondrial energy production, especially under conditions of high energy demand such as the increased workload induced by isoproterenol infusion in vivo. Interestingly, a down-regulation of mitochondrial gene expression has been reported in dystrophic skeletal muscles (22). Likewise, PGC-1α-null mice, which display defects of mitochondrial metabolism (23), have a reduced ability to tolerate β-adrenergic stimulation, similar to mdx mice (7). Therefore, it appears conceivable that the mitigation of the dystrophic cardiomyopathy observed in mdx/GC+/0 mice is mediated at least in part by improved mitochondrial function. Such a mechanism would appear to reconcile many findings. Indeed, it has been reported that augmented cGMP signaling prevents mitochondria-mediated cell death through reduced opening of the mitochondrial permeability transition pore (24). The opening of this pore was recently shown to be associated with dysregulated β-adrenergic receptor signaling and Ca2+ handling (25). In this regard, it has been reported that intracellular Ca2+is elevated in the dystrophin-deficient heart at all ages (26), whereas activation of the cGMP pathway has been shown to lower intracellular Ca2+ concentration through activation of cGMP-dependent protein kinase (PKG) (27).
Although the genetic and pharmacological approaches used to enhance cGMP signaling in the mdx heart resulted in similar protective effects, there are potential differences in their mechanisms of action that deserve some comment. PDE5 inhibition has been shown to attenuate increases in pulmonary arterial pressures and pulmonary vascular resistance (28–31), which could alter the cardiac workload. Other data suggest that sildenafil improves endothelial dysfunction, dilates coronary arteries, and inhibits platelet activation in patients with heart failure (32, 33). Sildenafil also significantly reduces exercise-induced ischemia (33), a potentially important pathogenetic mechanism in dystrophin-deficient skeletal muscle (10, 34). In fact, defects in cGMP signaling are also suggested from studies in dystrophin-deficient skeletal muscle (35, 36), and recently, Asai et al. (37) showed that treatment with tadalafil, another known PDE5 inhibitor, ameliorates contraction-induced skeletal muscle damage. Finally, sildenafil can cross the blood–brain barrier to inhibit brain PDE5A (38), which could theoretically affect sympathetic and parasympathetic outflow. Therefore, although the resting echocardiographic and hemodynamic parameters obtained in sildenafil-treated mice did not reveal any significant differences compared with placebo-treated animals, we cannot exclude the possibility that neurohumoral systemic effects of PDE5 inhibition had some influence on mdx hearts.
With respect to cardiomyocyte-specific effects of cGMP up-regulation, there may also be significant differences between the transgenic mdx/GC+/0 and sildenafil models. Indeed, we have shown that constitutive activation of guanylyl cyclase in our mdx/GC+/0 model chronically increases tissue cGMP levels. This is in agreement with our previous characterization of GC+/0 animals, in which the expression of the transgene was accompanied by increased guanylate cyclase activity and cGMP concentration in isolated cardiomyocytes compared with nontransgenic littermates (17). However, sildenafil treatments transiently increase cGMP tissue concentration, which peaks at ≈45 min after drug administration and returns to control levels within 90 min. Kukreja and coworkers have shown that, despite a short half-life in plasma, a single administration of sildenafil induces cardioprotective effects up to 24 h, suggesting it is not essential to sustain high plasma levels of sildenafil to induce the protective effects (31, 39, 40). Similarly, in this study, the enhanced protection of mdx cardiomyocytes against mechanical workload-induced sarcolemmal damage was documented 20 h after the last sildenafil injection. In fact, our gene expression data, namely the decreased expression of anf and sgcα1 genes after sildenafil administration, do suggest long-term cellular changes associated with cGMP signaling.
Another potential difference between the transgenic mdx/GC+/0 and sildenafil models relates to the compartmentation of cGMP signaling within cardiomyocytes. Sildenafil inhibits PDE5, which is specific for cGMP and exerts a specific spatiotemporal control on the pool of intracellular cGMP synthesized by soluble GC but not particulate GC (41). In our transgenic mdx/GC+/0 model, the transgene codes for the GC catalytic fragment of the natriuretic peptide receptor A, a soluble cytoplasmic protein that has constitutive GC activity (17). The exact subcellular localization of this engineered protein remains to be determined, but differences in the spatiotemporal distribution or kinetics of cGMP turnover within cardiac muscle cells may be responsible for the slight differences in the effects of the genetic and pharmacological approaches.
In conclusion, this study demonstrates that enhanced cGMP signaling improves contractile performance, sarcolemmal integrity, and mitochondrial metabolic status in dystrophin-deficient hearts. These findings are compatible with the notion that defects in the cGMP signaling pathway play a crucial role in the pathogenesis of the cardiomyopathy associated with dystrophin deficiency. Furthermore, our data indicate that evolution of the dystrophic cardiomyopathy can be modified independently of the pathology progressing in other muscle groups, because cGMP generated in a cardiomyocyte-restricted manner protects the dystrophin-deficient heart against stress-induced injury and from a progressive age-dependent loss of myocardial function. Because there are currently safe and well tolerated pharmacological means to enhance cGMP signaling, such drugs could provide the basis for a new therapeutic strategy based on PDE5 inhibition in the dystrophic heart. Further studies are required to determine whether PDE5 inhibition can delay, prevent, or even reverse the onset of dystrophic cardiomyopathies in DMD/BMD patients. It also appears warranted to evaluate the potential benefit of this treatment for patients suffering from the acquired forms of cardiomyopathy in which dystrophin abnormalities have been reported.
Materials and Methods
Reagents and Animals.
Sources of the chemical reagents and biological products used in this study have been reported (7, 8, 17, 42). Genetic up-regulation of the cGMP pathway in mdx mice was achieved by cross-breeding female mdx mice (The Jackson Laboratories), with heterozygous male C56Bl/6 mice expressing a catalytic fragment of the constitutively activated GC domain of the atrial natriuretic factor receptor, under the control of the cardiac-specific α-myosin heavy chain promoter as described (17). Because the dystrophin locus is on chromosome X, all males resulting from this breeding were dystrophin-deficient (mdx), and 50% were heterozygous for the GC transgene (mdx/GC+/0), as confirmed by Southern blot analysis and/or PCR (17). To avoid the possible artifactual influence of gene inactivation by insertion, all transgenic animals used for experiments were heterozygous for the transgene. Treatment with the PDE5 inhibitor, sildenafil (pure substance obtained from Pfizer), was initiated at 6 weeks of age in male mdx mice and continued over a 6-week period, with daily i.p. injections (0.7 mg/kg per day). Control animals were injected in the identical manner with equivalent volumes of the drug's saline vehicle. Treatments were begun before the onset of any known cardiomyopathy in mdx mice (43). All animal experiments were approved by the local ethics committee and performed according to the guidelines of the Canadian Council on Animal Care.
Functional and Metabolic Phenotyping of Isolated Working Hearts.
The procedures for in vivo working mouse heart perfusions in the semirecirculating mode have been described in detail (42). Briefly, working mouse hearts were perfused for 30 min with a semirecirculating modified Krebs–Henseleit buffer containing physiological concentrations of substrates (11 mM glucose, 0.8 nM insulin, 50 μM carnitine, 5 nM epinephrine, 1.5 mM lactate, 0.2 mM pyruvate, 0.7 mM oleate bound to 3% albumin, and 0.1 mM EDTA). Preload and afterload were respectively set at 12.5 and 40 mmHg to: (i) obtain similar cardiac power between mdx and mdx/GC+/0 for the metabolic studies and (ii) optimize perfusion conditions for the more advanced cardiomyopathy found in 20-week-old mdx mice. In each perfusion experiment, [U-13C3]pyruvate [initial molar percentage enrichment (MPE), 99%] was used to assess the contribution of carbohydrates to energy production and [1-13C18]oleate (initial MPE, 100%) for that of fatty acids (n = 4 per group). Definitions of the 13C terminology and detailed descriptions for: (i) measurements of the 13C enrichment and concentrations of CAC intermediates by gas chromatography coupled with mass spectrometry and (ii) calculations of flux ratios relevant to substrate selection for citrate synthesis have been published (42).
In Vivo Manipulation of Cardiac Workload and Quantification of Cardiomyocyte Sarcolemmal Damage.
Our previously described protocol was used to acutely increase cardiac workload in vivo and induce cardiomyocyte sarcolemmal injury (7) in mdx (n = 7), mdx/GC+/0 (n = 5), placebo (n = 5), and sildenafil- (n = 7) treated mdx mice. This protocol, which involves isoproterenol infusion (0.1 mg/kg) and bolus injection of Evans blue (5 μg/μl solution in saline; 5 μl/g body weight over 1 min) into the jugular vein, is sufficiently sensitive for detecting therapeutic effects (44, 45).
Echocardiography and Cardiac Hemodynamic Measurements.
After 6 weeks of daily treatment, the placebo (n = 7) and sildenafil- (n = 10) treated mdx mice were anesthetized with the aforementioned ketamine/xylazine mixture. Cardiac hemodynamics were measured by using a 1.4-F Millar micromanometer-tipped catheter (Millar Instruments) inserted into the left ventricle via the right carotid and through the aortic valve in a retrograde fashion. Data were collected and analyzed using IOX software (Emka Technologies). In a different set of experiments, placebo and sildenafil-treated mdx mice (n = 8 for each group) were subjected to two-dimensional guided M-mode echocardiography after anesthesia with 2.5% isoflurane (Forane) in 0.5 liter per min of oxygen. Left ventricular internal diameters were measured during diastole and during systole, along with septal and posterior wall thickness. Fractional shortening (percentage) and left ventricular mass (gram) were calculated from published equations (46). Mice were randomly selected for testing, and the echocardiographer was blinded to the mouse treatment status.
Analyses of mRNA and Protein Expression.
Total RNA was extracted and reverse-transcribed by using standard methods, followed by quantitative real-time PCR as described in ref. 8. Gene expression analyses were carried out in 10- to 12-week-old hearts freeze-clamped in the afternoon (light phase) to avoid any potential circadian variation. The following genes were examined: (i) anf, a marker of early cardiac remodeling (47) and (ii) sgcα1, a marker for activation of the cGMP pathway (18). Transcript levels were normalized to total RNA content. Immunohistochemistry and Western blot analysis were used to localize and quantify cardiac utrophin levels according to described standard methods (48).
cGMP Measurements.
Twenty-five milligrams of powdered frozen mouse heart tissue (n = 4–6 hearts), homogenized by sonication in 350 μl of 0.1 M HCl. cGMP was measured in acetylated supernatants using a RIA kit (Biomedical Technologies), with modifications to match protocols described by others using an ELISA (49).
Statistical Analysis.
Data are expressed as means ± SEM (n = 4–8 experiments). Statistical significance was defined as P < 0.05 using Student's unpaired t test or ANOVA (one- or two-way), followed by a Bonferroni selected-comparison test.
Supplementary Material
Acknowledgments.
We thank Johanne Bourdon for assistance in cardiac tissue analysis, Dr. Linda Yahiaoui for performance of utrophin Western blot analyses, Sylvie Picard for cGMP measurements, and Dr. Guy Charron for his expertise in animal breeding and genotyping. We thank Dr. Jean-Claude Tardif and Francine Poulin for their assistance in echocardiographic measurements. This work was supported by the Canadian Institutes of Health Research [Grants 9575 (to C.D.R.), 12743 (to B.J.P.), and 74460 (to C.F.D. and C.D.R.)]; the Muscular Dystrophy Association (B.J.P.); the Heart and Stroke Foundation of Canada (studentship to M.K.); and the National Heart, Lung, and Blood Institute (Grant HL-074259-01, to M.E.Y.). B.J.P. is a National Research Scholar of the Fonds de la Recherche en Santé Québec.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710595105/DCSupplemental.
References
- 1.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 2.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Yan J, Buzin CH, Towbin JA, Sommer SS. Mutations in the dystrophin gene are associated with sporadic dilated cardiomyopathy. Mol Genet Metab. 2002;77:119–126. doi: 10.1016/s1096-7192(02)00153-1. [DOI] [PubMed] [Google Scholar]
- 4.Vatta M, et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- 5.Badorff C, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 6.Sicinski P, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 7.Danialou G, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 8.Khairallah M, et al. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–129. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas GD, et al. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 12.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley RWR, et al. Sarcolemmal damage in dystrophin deficiency is modulated by synergistic interactions between mechanical and oxidative/nitrosative stresses. Am J Pathol. 2006;168:R704–R710. doi: 10.2353/ajpath.2006.050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bia BL, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 15.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 16.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 17.Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF. Expression of constitutively active guanylate cyclase in cardiomyocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem. 2003;278:47694–47699. doi: 10.1074/jbc.M309661200. [DOI] [PubMed] [Google Scholar]
- 18.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 19.Qian CN, Takahashi M, Kahnoski RJ, Teh BT. Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model. J Urol. 2003;170:994–997. doi: 10.1097/01.ju.0000080321.99119.df. [DOI] [PubMed] [Google Scholar]
- 20.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 22.Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arany Z, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Costa AD, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn JF, Radda GK. Total ion content of skeletal and cardiac muscle in the mdx mouse dystrophy: Ca2+ is elevated at all ages. J Neurol Sci. 1991;103:226–231. doi: 10.1016/0022-510x(91)90168-7. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler B, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 29.Tsai BM, et al. Differential effects of phosphodiesterase-5 inhibitors on hypoxic pulmonary vasoconstriction and pulmonary artery cytokine expression. Ann Thorac Surg. 2006;81:272–278. doi: 10.1016/j.athoracsur.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia S, Frantz RP, Severson CJ, Durst LA, McGoon MD. Immediate and long-term hemodynamic and clinical effects of sildenafil in patients with pulmonary arterial hypertension receiving vasodilator therapy. Mayo Clin Proc. 2003;78:1207–1213. doi: 10.4065/78.10.1207. [DOI] [PubMed] [Google Scholar]
- 31.Salloum FN, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol. 2008:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 32.Gori T, et al. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation. 2005;111:742–746. doi: 10.1161/01.CIR.0000155252.23933.2D. [DOI] [PubMed] [Google Scholar]
- 33.Halcox JP, et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- 34.Sander M, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kito S, Yamamoto M, Itoga E, Kishida T. Cyclic nucleotides in progressive muscular dystrophy. Eur Neurol. 1979;18:356–360. doi: 10.1159/000115103. [DOI] [PubMed] [Google Scholar]
- 36.Lau KS, et al. Skeletal muscle contractions stimulate cGMP formation and attenuate vascular smooth muscle myosin phosphorylation via nitric oxide. FEBS Lett. 1998;431:71–74. doi: 10.1016/s0014-5793(98)00728-5. [DOI] [PubMed] [Google Scholar]
- 37.Asai A, et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker DK, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 39.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 40.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 41.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khairallah M, et al. Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol. 2004;286:H1461–H1470. doi: 10.1152/ajpheart.00942.2003. [DOI] [PubMed] [Google Scholar]
- 43.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 44.Yue Y, Skimming JW, Liu M, Strawn T, Duan D. Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice. Hum Mol Genet. 2004;13:1669–1675. doi: 10.1093/hmg/ddh174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuda S, et al. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 46.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 47.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Waheed I, et al. Factors associated with induced chronic inflammation in mdx skeletal muscle cause posttranslational stabilization and augmentation of extrasynaptic sarcolemmal utrophin. Hum Gene Ther. 2005;16:489–501. doi: 10.1089/hum.2005.16.489. [DOI] [PubMed] [Google Scholar]
- 49.Suliman HB, et al. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.