Abstract
Rationale: Infection with rhinovirus (RV) triggers exacerbations of asthma and chronic obstructive lung disease.
Objectives: We sought to develop a mouse model of RV employing RV1B, a minor group serotype that binds to the low-density lipoprotein receptor.
Methods: C57BL/6 mice were inoculated intranasally with RV1B, replication-deficient ultraviolet (UV)-irradiated RV1B, or RV39, a major group virus.
Measurements and Main Results: Viral RNA was present in the lungs of RV1B-treated mice, but not in those exposed to UV-irradiated RV1B or RV39. Lung homogenates of RV-treated mice contained infectious RV 4 days after inoculation. RV1B exposure induced neutrophilic and lymphocytic airway inflammation, as well as increased lung expression of KC, macrophage-inflammatory protein-2, and IFN-α and IFN-β. RV1B-exposed mice showed airway hyperresponsiveness 1 and 4 days after inoculation. UV-irradiated RV1B induced modest neutrophilic airway inflammation and hyperresponsiveness 1 day after exposure. Both RV1B and UV-irradiated RV1B, but not RV39, increased lung phosphorylation of Akt. Confocal immunofluorescence showed colocalization of RV1B and phospho-Akt in the airway epithelium. Finally, pretreatment with the phosphatidylinositol 3-kinase inhibitor LY294002 attenuated chemokine production and neutrophil infiltration.
Conclusions: We conclude that RV1B induces airway inflammation in vivo. Evidence is presented that viral replication occurs in vivo and is required for maximal responses. On the other hand, viral replication was not required for a subset of RV-induced responses, including neutrophilic inflammation, airway hyperresponsiveness, and Akt phosphorylation. Finally, phosphatidylinositol 3-kinase/Akt signaling is required for maximal RV1B-induced airway neutrophilic inflammation, likely via its essential role in virus internalization.
Keywords: asthma, chronic obstructive pulmonary disease, Akt, low-density lipoprotein receptor
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Understanding of rhinovirus-induced exacerbations of asthma and chronic obstructive lung disease is incomplete, in part because of the absence of an animal model.
What This Study Adds to the Field
Rhinovirus-1B exposure induces airway inflammation and hyperresponsiveness in mice. Phosphatidylinositol 3-kinase/Akt signaling is required for maximal rhinovirus-induced airway inflammation.
Viral infections trigger nearly 80% of asthma exacerbations, and rhinovirus (RV) accounts for the majority of virus-induced exacerbations (1, 2). RV also accounts for a substantial percentage of chronic obstructive pulmonary disease (COPD) exacerbations (3, 4). Understanding of RV-induced exacerbations is incomplete, in part because of the absence of an animal model. Rhinovirus RNA has been detected by polymerase chain reaction (PCR) analysis in lower airway cells from volunteers experimentally infected with RV16 (5, 6) and RV capsid protein has been found in airway epithelial cells, albeit sporadically (6). However, RV has not been cultured from the lower airways of immunocompetent subjects, and therefore the extent to which RV infects or replicates in the lower airways of humans remains unclear.
RV, a member of the Picornaviridae family of viruses, is responsible for the majority of common colds. The virus is composed of an icosahedral protein capsid and a positive, single-stranded RNA genome. More than 100 serotypes of RV have been identified. These are divided into two groups on the basis of their cellular receptors. The major subgroup RVs (e.g., RV14, RV16, and RV39) comprise 90% of RV serotypes and attach to intercellular adhesion molecule (ICAM)-1 on the airway epithelial cell surface. In contrast, minor subgroup RVs (e.g., RV1B and RV2) attach to proteins of the low-density lipoprotein receptor (LDL-R) family, which consists of LDL-R, LDL-R–related protein, very low–density lipoprotein receptor, and apoER2. Despite these differences, RV16, a major group RV, and RV1B, a minor group RV, induce similar chemokine responses in human airway epithelial cells (7, 8).
Whereas species-specific variations in ICAM-1 prevent infection of mouse cells by major group RV, the LDL-R family of proteins is highly conserved between human and mouse, providing a possible means of infection for minor group viruses. Early studies examining the restriction of RV2 replication in a mouse fibroblast cell line suggested that these cells lack the intracellular molecules required for RV replication (9). However, it was subsequently shown that RV16 replicates in mouse cells after transfection of viral RNA (10). Additional studies have shown replication of RV1A in cultured mouse fibroblasts (11) and replication of RV1B in LA-4 mouse airway epithelial cells (12). We have also observed replication of RV1B in primary mouse tracheal epithelial cells as evidenced by detection of cytopathic effect, cytokine induction, and viral 3C protease expression with intact but not ultraviolet (UV)-inactivated virus (P. Jourdan, J. Burnet, and S. Johnston, unpublished data). It is therefore conceivable that RV1B, a minor group virus, infects mouse airways in vivo.
Numerous studies suggest a role for C-X-C chemokines with the neutrophil-attractant Glu-Leu-Arg (ELR) motif in the pathogenesis of asthma and COPD exacerbations (13–22). We have shown in airway epithelial cells that RV colocalizes with the p110β catalytic subunit of phosphatidylinositol (PI) 3-kinase and the serine-threonine kinase Akt in lipid rafts (23). ICAM-1 engagement induces phosphorylation of the PI 3-kinase p85 regulatory subunit, activation of PI 3-kinase, accumulation of 3-phosphorylated PI at the site of RV infection, and Akt phosphorylation (23, 24). Inhibition of PI 3-kinase and Akt blocked internalization of labeled RV into cells, and attenuated RV-induced NF-κB trans-activation and IL-8 expression (24). Finally, replication-deficient, UV-irradiated virus also elicited IL-8 expression, suggesting that viral replication was not required for the IL-8 response. On this basis, we hypothesize that RV1B exposure induces neutrophilic airway inflammation in mice, and that PI 3-kinase/Akt signaling, which is activated on binding of RV to the airway epithelial cell surface in vitro, is required for neutrophilic inflammation in vivo.
Some of the results of these studies have been previously reported in the form of an abstract (25).
METHODS
See the online supplement for additional details regarding all methods.
Animals
Six- to 8-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were housed in a pathogen-free area within the institutional animal care facility of the University of Michigan (Ann Arbor, MI).
Cell Culture
16HBE14o− human bronchial epithelial cells were provided by S. R. White (University of Chicago, Chicago, IL). HeLa and LA-4 mouse bronchial epithelial cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA).
Generation of RV Stocks
RV1B was generated from an infectious cDNA clone (12). RV39 was obtained from the ATCC. Viral stocks were concentrated and partially purified as described (24, 26). Virus was titered by infecting HeLa monolayers with serially diluted RV and assessing cytopathic effect. Fifty percent tissue culture infectivity doses (TCID50) were determined by the Spearman-Karber method (27). Purified RV1B was irradiated with UV light, using a CL-1000 crosslinker (UVP, Upland, CA) (26). Efficiency of irradiation was confirmed by reverse transcriptase (RT)-PCR (see below).
RV1B Exposure
16HBE14o− and LA-4 cells were serum-starved overnight and infected with RV1B (multiplicity of infection of 1.0 for 1 h at 33°C). Mice were anesthetized by intraperitoneal injection with ketamine and xylazine, and intranasally inoculated with 50 μl of RV1B (1 × 108 TCID50/ml) or an equal volume of sham HeLa cell lysate. In selected experiments, mice were given 50 μl of LY294002 (3 mg/kg; Sigma-Aldrich, St. Louis, MO) intranasally or dimethyl sulfoxide vehicle. One hour later, mice were intranasally inoculated with 20 μl of RV1B (2. 5 × 108 TCID50/ml) or an equal volume of sham HeLa cell lysate.
Immunoblotting
Proteins were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and transferred to nitrocellulose. Membranes were probed with antibodies against Ser473 phospho-Akt and total Akt (Cell Signaling Technology, Danvers, MA).
Presence of Viral RNA
RNA was extracted from lungs with TRI Reagent (Sigma-Aldrich) and analyzed for viral RNA by RT-PCR, as described (28). Cellular RNA was also collected by nasal lavage. Quantitative real-time PCR was performed with specific primers and probes for positive- and negative-strand (replicative) RV RNA, as well as 18S rRNA (29, 30).
Histology and Immunofluorescence Staining
Sections were stained with hematoxylin and eosin and examined by light microscopy, or incubated with RV1B antiserum (ATCC) and phospho-Akt (Cell Signaling Technology) and visualized by confocal fluorescence microscopy.
Determination of RV1B Infectivity
RV1B infectivity was assessed by homogenizing lungs from mice inoculated with RV1B, UV-irradiated RV1B, or sham treatment; overlaying this material onto confluent monolayers of HeLa cells; assessing cytopathic effects; and measuring viral RNA expression by PCR.
Lung Inflammatory Cells
Bronchoalveolar lavage (BAL), differential cell counts, and myeloperoxidase (MPO) activity assays were performed as described (31).
Cytokine/Chemokine Expression
Protein levels in cell-conditioned medium and lung homogenates were measured by ELISA (R&D Systems, Minneapolis, MN). After RNA extraction, lung IFN-α, -β, and -γ mRNA levels were measured by quantitative real-time PCR using specific primers and probes.
Measurement of Respiratory System Resistance
Mice were anesthetized, endotracheally intubated, and ventilated with a flexiVent (Scireq, Montreal, PQ, Canada). Airway responsiveness was assessed by measuring changes in resistance after administration of nebulized methacholine.
Data Analysis
Statistical significance was assessed by one- or two-way analysis of variance. The Student-Newman-Keuls test was used to pinpoint differences identified by analysis of variance.
RESULTS
RV1B Infection Increases Phosphatidylinositol 3-Kinase–dependent Chemokine Production in Human and Murine Airway Epithelial Cells
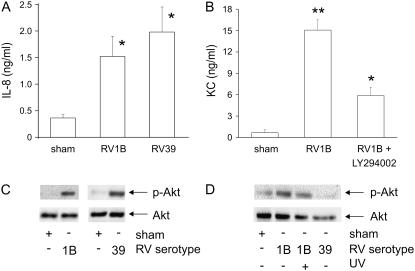
Confluent, serum-starved 16HBE14o− human airway epithelial cells were infected with minor serotype RV1B, major serotype RV39, or lysate from uninfected HeLa cells (sham) for 1 hour at 33°C. Conditioned medium was collected 48 hours after infection and examined for IL-8 expression by ELISA. RV1B and RV39 increased IL-8 expression to similar levels (Figure 1A). Using the same infection procedure, LA-4 murine airway epithelial cells were infected with RV1B and the murine IL-8 homolog KC/CXCL1 was examined by ELISA. RV1B increased KC expression nearly ninefold (Figure 1B). Pretreatment with the PI 3-kinase chemical inhibitor LY294002 attenuated RV1B-induced neutrophil chemoattractant expression in murine LA-4 cells, as it does in RV39-stimulated human airway epithelial cells (24). RV infection increased phosphorylation of Akt, a downstream effector protein of PI 3-kinase, in both human and murine airway epithelial cells (Figures 1C and 1D). Treatment with RV39 had no effect on LA-4 cell Akt phosphorylation (Figure 1D) or KC expression (data not shown). These data demonstrate that RV1B infection induces PI 3-kinase–dependent production of neutrophil chemoattractants in both murine and human airway epithelial cells.
Figure 1.
Human rhinovirus 1B (RV1B) infection increases chemokine production and Akt phosphorylation in human and murine airway epithelial cells. (A) 16HBE14o− human airway epithelial cells were infected with major group serotype RV39 or minor group serotype RV1B and conditioned medium was collected 48 hours postinfection and examined for IL-8/CXCL8 expression. (B) LA-4 murine airway epithelial cells were pretreated for 1 hour with LY294002 (10 μM) or dimethyl sulfoxide vehicle control and then infected with RV1B or mock-infected (sham) and murine IL-8 homolog, KC/CXCL1 was examined 48 hours postinfection. Bars represent the SEM for three experiments (*significantly different from sham, P < 0.05; **significantly different from sham and RV1B + LY294002, P < 0.05; one-way analysis of variance [ANOVA]). 16HBE14o− (C) and LA-4 cells (D) were infected with RV1B or RV39 for 10 minutes and examined for phosphorylation of Akt. Immunoblots shown are typical of three separate experiments. UV = ultraviolet.
Viral RNA Is Detectable in the Lungs of RV1B-treated Mice
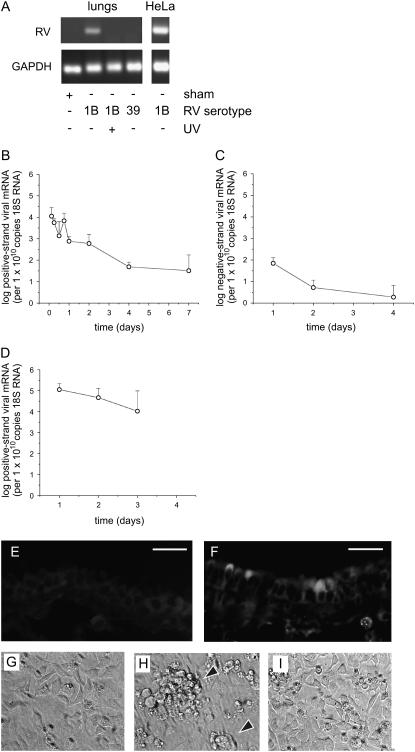
Female C57BL/6 mice were anesthetized and inoculated with RV1B (50 μl at 1 × 108 TCID50/ml) by intranasal instillation. One day after inoculation, mice were killed and the lungs were analyzed by RT-PCR for the presence of viral RNA. Selected mice were also inoculated with replication-deficient UV-irradiated RV1B or RV39. Viral RNA was detected after RV1B inoculation (Figure 2A). No viral RNA was detected in mice infected with UV-irradiated RV1B or RV39, a major group serotype. Positive- and negative-strand viral RNAs were also measured by quantitative PCR. Negative-strand viral RNA is generated only during viral replication, and therefore is suggestive of RV1B replication. Although there was a progressive decline in the level of positive-strand viral RNA with time, RV1B-exposed mice demonstrated the presence of viral RNA up to 7 days after inoculation, and there was a shallow but statistically significant peak of viral RNA expression 18 hours after RV exposure (Figure 2B), suggestive of viral replication. Consistent with this, a modest amount of negative-strand RNA was detectable up to 4 days after inoculation (Figure 2C). No negative-strand RNA was detectable in the viral preparation. Nasal aspirates of RV1B-treated mice showed rhinoviral RNA up to 3 days after exposure (Figure 2D). No viral RNA was detected in mice treated with uninfected HeLa cell lysate (sham inoculation) or UV-irradiated RV1B (data not shown).
Figure 2.
Viral RNA is detectable in the lungs of RV1B-treated mice. (A) Female C57BL/6 mice were inoculated with RV1B by intranasal instillation and lungs were examined by RT-PCR for viral RNA 1 day postinoculation. Mice were also exposed to ultraviolet (UV)-irradiated replication-deficient RV1B or RV39, a major group virus. As a positive control, HeLa cells were infected for 1 hour with RV1B at a multiplicity of infection of 10. RNA was extracted 16 hours postexposure and analyzed for viral RNA. Blots shown are typical of three separate experiments. GAPDH = glyceraldehyde-3-phosphate dehydrogenase. (B and C) Lungs of RV1B-inoculated mice were examined for positive-strand (B) and negative-strand (replicative) (C) rhinovirus (RV) RNA by quantitative PCR. Although positive-strand viral RNA decreased steadily after inoculation, viral RNA was detected up to 7 days postinoculation. There was a small but significant increase in positive-strand viral RNA copy number between the 12- and 18-hour time points (P = 0.043, one-way analysis of variance). We also detected a modest amount of negative-strand viral RNA, consistent with viral replication (n = 4–6, geometric mean ± SEM). (D) Positive-strand viral RNA was detected in the nasal washes of RV1B-inoculated mice up to 3 days after exposure (n = 3, geometric mean ± SEM). (E and F) One day postexposure, lungs from sham-inoculated (E) or RV1B-inoculated (F) mice were formalin-fixed and probed with purified RV1B antiserum. Specific staining for RV1B was noted in some but not all central airways. Scale bars: 20 μm. (G–I) Supernatants from homogenized mouse lungs that were sham inoculated (G), inoculated with RV1B (H), or inoculated with UV-irradiated RV1B (I) were overlaid onto confluent HeLa cell monolayers and examined for cytopathic effect (arrowheads). Images shown are typical of three separate experiments (original magnification, ×100).
Lungs were formalin-fixed and paraffin-embedded 1 day postexposure, and sections were stained for RV1B. Specific staining was seen in the airway epithelial cells of mice inoculated with RV1B but not in those of mice treated with an equal volume of uninfected HeLa cell lysate (sham inoculation) (Figures 2E and 2F). RV1B staining was confined to a subset of the large airways. Positive staining was observed in groups of cells in close proximity, suggesting that RV1B maintains its infectivity in vivo.
We also homogenized lungs 1 to 4 days after exposure and overlaid clarified supernatant containing RV1B on confluent HeLa cell monolayers. HeLa cells were examined for cytopathic effect, and HeLa cell lysates were examined for viral RNA by real-time PCR. Lung homogenate from RV1B-treated mice induced HeLa cytopathic effects up to 4 days after RV exposure, whereas homogenates from UV-irradiated RV1B- or sham-inoculated mice did not (Figures 2G–2I, and Table 1). Finally, viral RNA was detectable in HeLa cell lysates overlaid with lung homogenates from RV1B-treated mice.
TABLE 1.
EFFECTS OF LUNG HOMOGENATES FROM RHINOVIRUS-EXPOSED MICE ON HeLa CELL MONOLAYERS
| Exposure Agent | Time Postexposure (d) | Cytopathic Effect* | RNA (cycle number) | 18S RNA (cycle number) |
|---|---|---|---|---|
| RV1B | 1 | +++ | 28.98 | 19.84 |
| 1 | +++ | 29.02 | 20.40 | |
| 2 | ++++ | 34.1 | 20.07 | |
| 2 | ++++ | 35.0 | 20.4 | |
| 4 | ++++ | 24.6 | 20.05 | |
| 4 | ++++ | 22.1 | 19.51 | |
| UV-RV1B | 1 | ++ | 42.7 | 19.8 |
| 1 | ++ | 41.9 | 19.84 | |
| 2 | + | — | 20.27 | |
| 2 | + | — | 20.02 | |
| 4 | + | — | 19.05 | |
| 4 | + | — | 18.73 |
Definition of abbreviations: RV1B = minor group rhinovirus serotype; UV-RV1B = replication-deficient ultraviolet-irradiated RV1B.
Cells were examined for cytopathic effect, and cell lysates were examined for viral RNA by real-time polymerase chain reaction.
+, observed only in wells treated with 1:2 dilution of lung homogenates; ++, observed only in wells treated with 1:2 and 1:5 dilutions of lung homogenates; +++, observed only in well treated with 1:2, 1:5, and 1:10 dilutions of lung homogenates; ++++, observed in wells treated with 1:2, 1:5, 1:10, and 1:20 dilutions of lung homogenates; —, no detectable RNA.
RV1B Exposure Induces Airway Inflammation
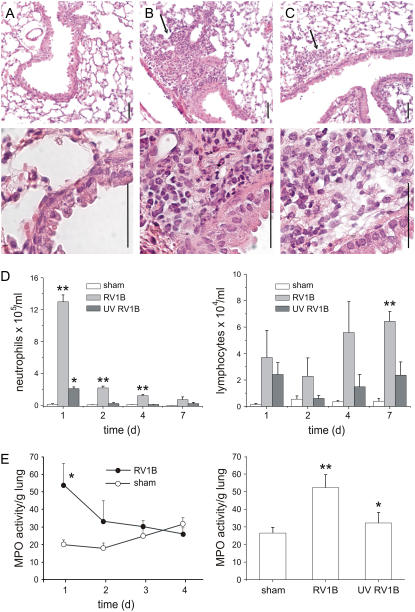
Formalin-fixed, paraffin-embedded lungs harvested 1 day postinoculation were stained with hematoxylin and eosin. Sham-inoculated mice showed no inflammation (Figure 3A). However, RV1B-exposed mice showed neutrophilic and monocytic inflammation around some large airways (Figure 3B). The lungs of mice inoculated with replication-deficient, UV-irradiated RV1B showed some evidence of neutrophilic inflammation, but affected areas were less numerous and smaller in size (Figure 3C).
Figure 3.
RV1B inoculation increases airway inflammation. Formalin-fixed, paraffin-embedded lungs harvested 1 day after viral exposure were stained with hematoxylin and eosin. (A) Sham-inoculated mice showed no inflammation. (B) RV1B-exposed mice demonstrated airway inflammation, indicated by arrows. (C) Mice exposed to ultraviolet (UV)-irradiated RV1B also showed evidence of inflammation (arrows). Scale bars: 50 μm. Images shown are typical of three separate experiments. (D) Bronchoalveolar lavage (BAL) was performed on mice 1 day postexposure and inflammatory cells were counted. Rhinovirus (RV)-exposed mice demonstrated increases in neutrophil (left) and lymphocyte (right) infiltration in the BAL fluid. (E) Left: Myeloperoxidase (MPO) activity was increased in RV1B-exposed mice 1 day postinoculation and declined to sham levels on Days 2 through 4. Right: In a separate experiment, mice exposed to UV-irradiated RV1B showed an intermediate increase in BAL neutrophil percentage and MPO activity. (Columns and error bars represent means ± SEM for three to nine mice. *Significantly different from sham, P < 0.05; **significantly different from sham and UV RV1B, P < 0.05; one-way analysis of variance.)
Neutrophil infiltration was quantified by BAL counts as well as lung MPO activity. One day postexposure, RV1B-infected mice showed a significant increase in BAL neutrophils compared with sham-infected animals (Figure 3D, left). The mean percentage of BAL neutrophils increased from 8% (sham inoculation) to 33% (RV1B). Interestingly, mice exposed to UV-irradiated virus demonstrated a moderate increase in BAL neutrophils compared with sham-exposed animals. The number of BAL neutrophils sharply declined 2 days after RV1B exposure. Similar to BAL counts, MPO activity was increased 1 day postexposure and declined thereafter (Figure 3E, left). Further, mice inoculated with UV-irradiated RV demonstrated a level of MPO activity intermediate between intact RV1B and sham inoculation (Figure 3E, right), consistent with the lung histology. Finally, RV1B-treated animals also showed increases in BAL lymphocyte counts (Figure 3D, right). Lymphocyte counts declined on Day 2 and then increased on Days 4 and 7 after exposure.
RV1B Increases Expression of Chemokines in Vivo
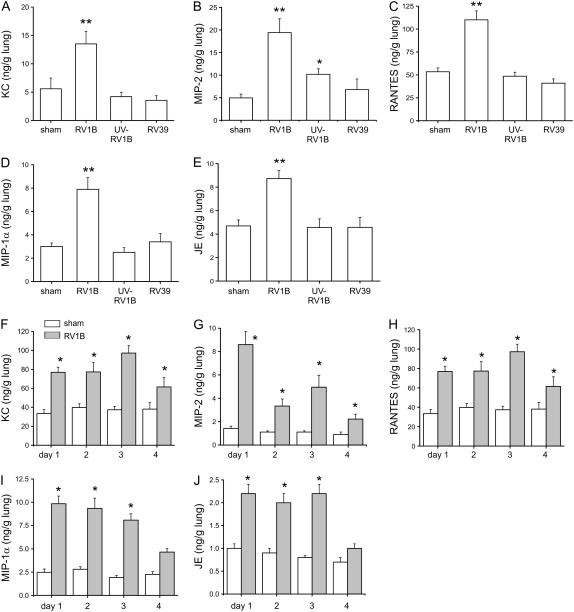
Mice were inoculated with sham HeLa cell lysate, RV1B, UV-irradiated RV1B, or RV39. KC, macrophage-inflammatory protein (MIP)-2/CXCL2-3, RANTES (regulated upon activation, T-cell expressed and secreted)/CCL5, MIP-1α/CCL3, and JE/CCL2 were increased after RV1B exposure compared with sham controls (Figures 4A–4E). Inoculation with UV-irradiated RV1B did not induce chemokine production above sham except in the case of the neutrophil chemoattractant MIP-2, the level of which was significantly different from both sham and RV1B. Inoculation with RV39 did not induce chemokine or cytokine production. We examined the time course of chemokine production up to 4 days after exposure to HeLa cell lysate or RV1B. Increases in KC, MIP-2, RANTES, MIP-1α, and JE protein expression were each sustained for at least 3 days after treatment (Figures 4F–4J).
Figure 4.
RV1B increases lung chemokine production. (A–E) Mice were exposed to RV1B, replication-deficient ultraviolet (UV)-irradiated RV1B, or major group virus RV39, and chemokines were analyzed 1 day postexposure. KC/CXCL1, RANTES (regulated upon activation, T-cell expressed and secreted)/CCL5, macrophage-inflammatory protein (MIP)-1α/CCL3, and JE/CCL2 were increased with RV1B exposure, but not after inoculation with UV-irradiated RV1B or RV39. Mice inoculated with UV-irradiated RV1B showed an intermediate increase in MIP-2/CXCL2-3 expression. (F–J) Chemokine production was measured for 4 days after RV1B exposure. RV1B increased KC, MIP-2, RANTES, MIP-1α, and JE expression. (Error bars represent the SEM for 6–17 mice; *significantly different from sham, P < 0.05; **significantly different from sham and UV RV1B, P < 0.05; one-way analysis of variance.)
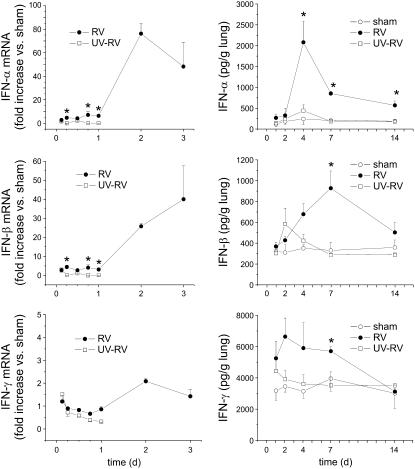
RV1B Increases Expression of Interferons In Vivo
Mice were exposed to sham HeLa cell lysate, RV1B, or UV-irradiated RV1B. RV1B-exposed mice, but not animals exposed to sham HeLa cell lysate or UV-irradiated RV1B, demonstrated significant increases in lung IFN-α and -β mRNA and protein expression (Figures 5A and 5B), consistent with the presence viral RNA (see above).
Figure 5.
RV1B increases lung interferon production. Mice were exposed to HeLa cell lysate (sham inoculation), RV1B, or replication-deficient ultraviolet (UV)-irradiated RV1B, and interferon mRNA (left) and protein abundance (right) were analyzed 1–14 days postexposure. Relative to sham-inoculated mice, RV1B exposure increased IFN-α and IFN-β expression. (Data represent means ± SEM for three mice; *significantly different from sham inoculation and UV-irradiated RV, P < 0.05; one-way analysis of variance.)
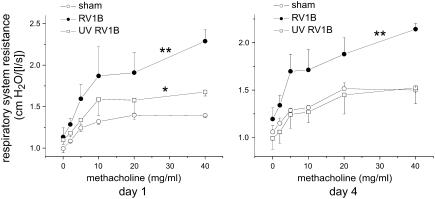
RV1B Exposure Increases Airway Responsiveness
At 1 and 4 days postinoculation, mice exposed to HeLa cell lysate, RV1B, or UV-irradiated RV1B were anesthetized and a cannula was inserted into the trachea for mechanical ventilation. Increasing doses of nebulized methacholine were given and respiratory system resistance was measured. One day after exposure, mice infected with RV1B demonstrated a significant increase in cholinergic airway responsiveness compared with sham mice (Figure 6). Mice exposed to UV-irradiated RV1B exhibited an intermediate state of airway responsiveness, which was significantly increased compared with sham controls. Four days after exposure, mice treated with RV1B, but not UV-irradiated RV1B, maintained a state of airway hyperresponsiveness (Figure 6).
Figure 6.
RV1B infection increases airway cholinergic responsiveness. Mice were anesthetized and endotracheally intubated, and changes in respiratory system resistance to nebulized methacholine were measured with the flexiVent system (Scireq, Montreal, PQ, Canada). Mice were studied either 1 day (left) or 4 days (right) after viral exposure. Mice inoculated with RV1B, compared with sham-inoculated mice, demonstrated airway cholinergic responsiveness that was present 1 day after exposure and persisted to 4 days after exposure. One day after exposure, mice given ultraviolet (UV)-irradiated RV1B exhibited an intermediate state of airway responsiveness that was significantly increased compared with sham controls. (Data represent means ± SEM for three mice; *significantly different from sham inoculation, P < 0.05; **significantly different from sham and UV-irradiated RV1B; P < 0.05, two-way analysis of variance.)
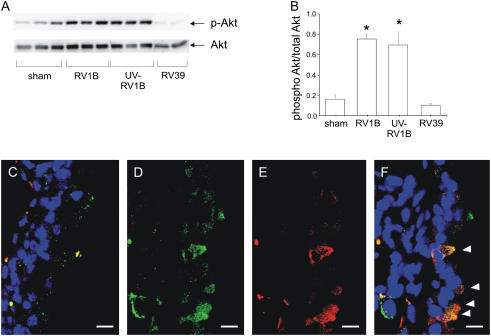
PI 3-Kinase/Akt Signaling Is Required for RV1B-induced Airway Inflammation
Whole lung homogenates from mice were collected 1 day postexposure. Phosphorylation of Akt, a downstream effector of PI 3-kinase, was increased in mice treated with RV1B or UV-irradiated RV1B, compared with sham controls or RV39-treated mice (Figures 7A and 7B). Confocal immunofluorescence microscopy showed colocalization of RV1B and phosphorylated Akt in the airways of RV1B-infected but not sham-infected mice (Figures 7C–7F).
Figure 7.
A phosphatidylinositol 3-kinase downstream target, Akt, is activated in RV1B- and ultraviolet (UV)-RV1B–exposed mice. Whole lung homogenates collected 1 day postexposure were analyzed for Ser473 Akt phosphorylation and total Akt expression. (A) Exposure to RV1B and UV-irradiated RV1B each increased Akt phosphorylation compared with sham inoculation (representative blot shown). (B) Densitometry showing RV1B- or UV RV1B–induced increases in Akt phosphorylation when compared with total Akt. Error bars represent the SEM for six mice (*significantly different compared with sham and RV39, P < 0.05; one-way analysis of variance). (C–F) RV1B and phospho-Akt colocalize in airway epithelial cells. Confocal fluorescence images demonstrate staining for RV1B (green), phospho-Akt (red), and airway nuclei (blue). For each image, the airway lumen is oriented to the right, and scale bars represent a length of 10 μm. (C) A merged image from a large airway of a sham-inoculated mouse. There is no specific staining of the airway epithelium. (D), (E), and (F) show green, red, and merged images, respectively, of a large airway from an RV1B-exposed mouse. Note orange-appearing colocalization of RV1B and phosphorylated Akt in four individual airway epithelial cells (arrowheads). Images shown are typical of three separate experiments.
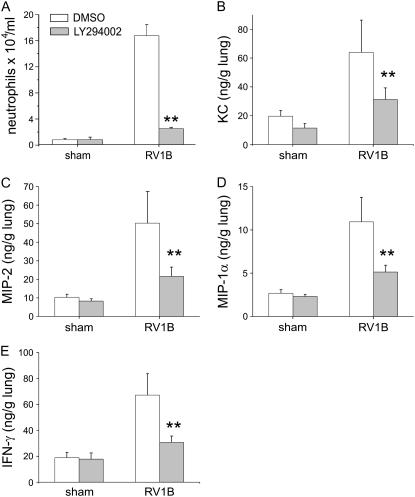
To determine whether PI 3-kinase, the upstream activator of Akt, is required for RV-induced responses, RV1B- or sham-inoculated mice were pretreated with LY294002 or vehicle, as described previously (32, 33). Mice received LY294002 (3 mg/kg) in 50 μl of dimethyl sulfoxide intranasally 1 hour before inoculation. Bronchoalveolar lavage was performed 1 day after viral exposure. LY294002 pretreatment decreased the percentage of neutrophils in BAL fluid in a dose-dependent manner (Figure 8A). LY294002 significantly reduced RV1B-induced KC, MIP-2, MIP-1α, and IFN-γ production (Figures 8B–8F). Taken together, these data suggest that PI 3-kinase is required for RV1B-induced neutrophil infiltration and maximal chemokine production in the lung.
Figure 8.
Phosphatidylinositol 3-kinase is required for maximal RV1B-induced chemokine production in vivo. Mice were pretreated with LY294002 (3 mg/kg body weight) or vehicle (dimethyl sulfoxide [DMSO]) and exposed to RV1B or sham treatment 1 hour later. (A) One day postexposure, LY294002 decreased the bronchoalveolar lavage neutrophil percentage. (B–E) LY294002 attenuated RV1B-induced KC, MIP-2, MIP-1α, and IFN-γ expression, respectively. (Error bars represent the SEM for eight or nine mice; **significantly different compared with RV1B and DMSO, P < 0.05; one-way analysis of variance.)
DISCUSSION
Viral infections trigger nearly 80% of asthma exacerbations, and RV accounts for the majority of virus-induced exacerbations (1). RV is also an important trigger of COPD exacerbations (3, 4). In the respiratory tract, RV replicates mainly in the ciliary epithelial cells of the nasal mucosa and, to a lesser extent, oral cavity and throat (34). In the common cold, typical symptoms such as coryza and cough climax on Day 2 or 3 and usually resolve by Day 5, although occasionally they may persist for longer. However, little is known about infection of the lower respiratory tract with RV. Until recently, rhinoviruses had not been reliably cultured from lower airway secretions (35). RV RNA has been detected by PCR in lower airway cells from volunteers experimentally infected with RV16 (5, 6), and rhinovirus capsid protein has been found in airway epithelial cells, albeit sporadically (6). Together these findings suggest that rhinoviruses can grow in the lower airways, although the extent of RV replication in these locations is unknown.
Because of species-specific variations in the ICAM-1 D1 extracellular immunoglobulin domain, mouse models of major group RV have not been implemented. However, the LDL-R family of proteins is highly conserved between human and mice, providing a possible means of infection for minor group viruses. In the present article, we show evidence that RV1B infects mouse airway epithelial cells in vivo. After intranasal inoculation with RV1B, we isolated positive-strand and negative (replicative)-strand viral RNAs from the lungs of C57BL/6 mice up to 7 and 4 days after exposure, respectively. We also detected RV1B protein in airway epithelial cells 1 day after inoculation. Although specific to the airway epithelium, RV1B protein expression was patchy and limited to the larger airways, similar to patients experimentally infected with RV16 (6). We also showed that lung homogenate from RV1B-exposed mice infects HeLa cells. RV1B exposure induced airway inflammation, as demonstrated by lung histology, increased BAL neutrophils and lymphocytes, and increased MPO activity. RV1B also induced the production of KC, MIP-2, RANTES, MIP-1α, and JE. The observed neutrophilic inflammation is similar to that found in human subjects after experimental RV16 infection (13, 15, 16). Neutrophil number, IL-8, and epithelial-derived neutrophil activating peptide-78 are also increased in the sputum and airways of patients with exacerbations of asthma (17, 18) and COPD (19–22). Airway inflammation was accompanied by a functional state of hyperresponsiveness that persisted 4 days after viral exposure. RV1B exposure induced a robust interferon response, further evidence of viral replication (36). Finally, inoculation with UV-irradiated virus had significantly reduced effects on airway inflammation, cytokine expression, and methacholine responsiveness compared with intact virus. Together, these data suggest that mouse lower airways may be infected with RV1B.
On the other hand, several observations speak against infection. First, although the extent and time course of viral replication in the human lung is unknown, the steep reduction in viral RNA we observed is inconsistent with the time course of viral replication in the upper respiratory tract of humans (37). Second, positive staining of the airway epithelium with RV1B antiserum does not prove replicative infection; the use of an antibody targeting nonstructural viral proteins would better address this issue. Third, because UV irradiation may partially inhibit picornavirus attachment (38), the effects of viral irradiation on RV-induced responses may overestimate the role of viral replication, and may instead represent an inhibition of RV binding to the airway epithelium.
Moreover, it should be noted that exposure to UV-irradiated virus, but not sham HeLa cell lysate, caused modest airway neutrophilic inflammation and short-lived airway cholinergic responsiveness. In addition, UV-irradiated RV induced the production of a mouse IL-8 homolog, MIP-2, providing a possible mechanism for the observed neutrophilic inflammation. Finally, UV-irradiated virus was sufficient to induce airway epithelial cell Akt phosphorylation (see below). These data suggest that early events before viral replication, for example, viral attachment and internalization, may be sufficient for a subset of RV-induced epithelial cell responses. Because, as noted above, UV irradiation may partially inhibit picornavirus attachment (38), and therefore UV inactivation, experiments may underestimate the sufficiency of virus attachment and internalization for cellular responses. Numerous studies have demonstrated the sufficiency of UV-irradiated virus to induce IL-8 expression in cultured airway epithelial cells (24, 39–42). Bafilomycin, an inhibitor of vacuolar proton ATPases, which promotes the low endosomal pH needed for viral uncoating, decreases RV14-induced ICAM-1 but not IL-8 expression in human tracheal epithelial cells (40). If indeed events before viral replication such as attachment and internalization were sufficient for a subset of RV-induced epithelial cell responses, this could explain how RV enhances lower airway inflammation in the absence of abundant viral replication (35).
RV attachment and endocytosis promote phosphorylation of the p85 regulatory subunit of PI 3-kinase, as well as activation of PI 3-kinase and Akt phosphorylation, in cultured human bronchial epithelial cells (24). In the present study, we found that RV1B colocalizes with phosphorylated Akt in the airway epithelium of exposed mice, and increases the phosphorylation of Akt in whole lung extracts. Pretreatment of RV1B-infected mice with the PI 3-kinase chemical inhibitor LY294002 decreased BAL neutrophils and lung KC, MIP-2, MIP-1α, and IFN-γ production. Administration of UV-irradiated replication-deficient virus also increased Akt phosphorylation, but induced only modest neutrophilia and lung KC expression. Taken together, these data suggest that activation of PI 3-kinase/Akt signaling is required but not sufficient for maximal RV-induced airway inflammation. We have previously shown in cultured human bronchial epithelial cells that PI 3-kinase activity is required for viral internalization (24), and therefore this is likely the mechanism by which LY294002 blocks RV1B-induced airway responses. PI 3-kinase may therefore represent a therapeutic target for RV-induced exacerbations of chronic airway disease.
As expected, RV39, a major subgroup RV, failed to induce airway inflammation in C57BL/6 mice, likely due to species-specific variations in ICAM-1. We therefore could not compare the signaling events and inflammatory events initiated by major group RV with those found in the present study after minor group (RV1B) exposure. However, infection with RV1B and RV39 induced similar levels of Akt phosphorylation and IL-8 expression in cultured 16HBE14o− human bronchial epithelial cells, and inhibition of PI 3-kinase blocked RV1B-induced IL-8 expression (data not shown), just as it blocks RV1B-induced KC expression in mouse cells. Although the downstream signaling events after ligation of LDL-R have not been extensively studied, LDL-R family members bind ligands and internalize them for lysosomal degradation by clathrin-mediated endocytosis, similar to ICAM-1 (43). Ligation of LDL-R by apoprotein E and LDL induces Akt activation (44). Lipoprotein particles induce NADPH oxidase-mediated production of superoxide in endothelial cells via an LDL-R family receptor (45), as has been reported after RV16 infection of A549 cells (46). Finally, one study has shown that RV1B and RV16, a major group serotype, induce nearly identical patterns of gene expression in primary cultured airway epithelial cells (47). Thus, there are ample data suggesting that major and minor subgroup RVs elicit similar airway epithelial cell signaling pathways and inflammatory responses.
We conclude that RV1B exposure induces airway inflammation and hyperresponsiveness in C57BL/6 mice. Although our results are suggestive, we cannot conclusively state that the observed airway changes are due to replicative infection, rather than simply binding and endocytosis of the virus. Indeed, nonreplicative UV-irradiated virus was sufficient to induce a subset of RV-induced responses. Nevertheless, our model, especially when combined with established animal models of asthma, COPD, and cystic fibrosis, could provide important insight into the pathogenesis of RV-induced exacerbations of chronic airway disease. Finally, on the basis of our observation that activation of PI 3-kinase/Akt signaling is required for maximal RV-induced neutrophilic airway inflammation, future studies examining the role of PI 3-kinase inhibitors in the treatment of RV-induced airway disease may be warranted.
Supplementary Material
Acknowledgments
The authors thank S. R. White (University of Chicago) for the gift of 16HBE14o− human bronchial epithelial cells, and N. W. Bartlett and R. P. Walton (Department of Respiratory Medicine, National Heart and Lung Institute, Imperial College London) for advice concerning quantitative real-time PCR.
Supported by NIH grants HL81420 and HL82550 (M.B.H.) and AI036302 (N.W.L.).
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: D.C.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. U.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Q.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.T.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.C.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.W.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.L.J. is a holder of patents relating to the use of interferons in this therapeutic area, and he consults for and holds share options in Synairgen. M.B.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, Ghildyal R, Bardin P. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy 2005;35:672–678. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:167–173. [DOI] [PubMed] [Google Scholar]
- 4.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000;181:1875–1884. [DOI] [PubMed] [Google Scholar]
- 6.Mosser AG, Vrtis R, Burchell L, Lee W-M, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med 2005;171:645–651. [DOI] [PubMed] [Google Scholar]
- 7.Edwards MR, Johnson MW, Johnston SL. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol 2006;34:616–624. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, Johnston SL. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy 2001;31:1060–1066. [DOI] [PubMed] [Google Scholar]
- 9.Yin FH, Lomax NB. Host range mutants of human rhinovirus in which nonstructural proteins are altered. J Virol 1983;48:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JR, Racaniello VR. Changes in rhinovirus protein 2C allow efficient replication in mouse cells. J Virol 2003;77:4773–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reithmayer M, Reischl A, Snyers L, Blaas D. Species-specific receptor recognition by a minor-group human rhinovirus (HRV): HRV serotype 1A distinguishes between the murine and the human low-density lipoprotein receptor. J Virol 2002;76:6957–6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuthill TJ, Papadopoulos NG, Jourdan P, Challinor LJ, Sharp NA, Plumpton C, Shah K, Barnard S, Dash L, Burnet J, et al. Mouse respiratory epithelial cells support efficient replication of human rhinovirus. J Gen Virol 2003;84:2829–2836. [DOI] [PubMed] [Google Scholar]
- 13.Grunberg K, Smits HH, Timmers MC, De Klerk EPA, Dolhain RJ, Dick EC, Hiemstra PS, Sterk PJ. Experimental rhinovirus 16 infection: effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med 1997;156:609–616. [DOI] [PubMed] [Google Scholar]
- 14.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds: inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med 1998;158:1178–1184. [DOI] [PubMed] [Google Scholar]
- 15.Fleming H, Little F, Schnurr D, Avila P, Wong H, Liu J, Yagi S, Boushey H. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med 1999;160:100–108. [DOI] [PubMed] [Google Scholar]
- 16.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med 2000;162:2226–2231. [DOI] [PubMed] [Google Scholar]
- 17.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med 2000;161:769–774. [DOI] [PubMed] [Google Scholar]
- 18.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma. clinical and biologic significance. Am J Respir Crit Care Med 2000;161:1185–1190. [DOI] [PubMed] [Google Scholar]
- 19.Bhowmik A, Seemungal TAR, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000;55:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gompertz S, O'Brien C, Bayley DL, Hill SL, Stockley RA. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 2001;17:1112–1119. [DOI] [PubMed] [Google Scholar]
- 21.Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:349–355. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy Neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:968–975. [DOI] [PubMed] [Google Scholar]
- 23.Bentley JK, Newcomb DC, Goldsmith AM, Jia Y, Sajjan US, Hershenson MB. Rhinovirus activates IL-8 expression via a Src/p110β PI 3-kinase/Akt pathway in human airway epithelial cells. J Virol 2007;81:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcomb DC, Sajjan U, Nanua S, Jia Y, Goldsmith AM, Bentley JK, Hershenson MB. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J Biol Chem 2005;280:36952–36961. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb DC, Sajjan U, Bartlett NW, Walton RP, Jourdan PA, Burnet JC, Rodriguez ML, Jia Y, Tsai WC, Bentley JK, et al. Murine rhinovirus 1B infection causes neutrophilic airway inflammation [abstract]. Proc Am Thorac Soc 2006;3:A281. [Google Scholar]
- 26.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 via increased NF-κB-mediated transcription. J Biol Chem 1999;274:9707–9720. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SL, Tyrrell DAJ. Rhinoviruses. In: Lennette EH, Schmidt NJ, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. Washington, DC: American Public Health Association; 1997. pp. 553–563.
- 28.Sajjan US, Jia Y, Newcomb DC, Bentley JK, Lukacs NW, LiPuma JJ, Hershenson MB. Influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J 2006;20:2121–2123. [DOI] [PubMed] [Google Scholar]
- 29.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med 2006;12:1023–1026. [DOI] [PubMed] [Google Scholar]
- 30.Komurian-Pradel F, Perret M, Deiman B, Sodoyer M, Lotteau V, Paranhos-Baccala G, Andre P. Strand specific quantitative real-time PCR to study replication of hepatitis C virus genome. J Virol Methods 2004;116:103–106. [DOI] [PubMed] [Google Scholar]
- 31.Tsai WC, Rodriguez ML, Young KS, Deng JC, Thannickal VJ, Tateda K, Hershenson MB, Standiford TJ. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am J Respir Crit Care Med 2004;170:1331–1339. [DOI] [PubMed] [Google Scholar]
- 32.Kwak Y-G, Song CH, Yi HK, Hwang PH, Kim J-S, Lee KS, Lee YC. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 2003;111:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol 2005;5:495–502. [DOI] [PubMed] [Google Scholar]
- 34.Cate TR, Couch RB, Johnson KM. Studies with rhinoviruses in volunteers: production of illness, effect of naturally acquired antibody, and demonstration of a protective effect not associated with serum antibody. J Clin Invest 1964;43:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halperin SA, Eggleston PA, Hendley JO, Suratt PM, Groschel DH, Gwaltney JM. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis 1983;128:806–810. [DOI] [PubMed] [Google Scholar]
- 36.Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature 2003;424:516–523. [DOI] [PubMed] [Google Scholar]
- 37.Hendley JO, Gwaltney JM. Viral titers in nasal lining fluid compared to viral titers in nasal washes during experimental rhinovirus infection. J Clin Virol 2004;30:326–328. [DOI] [PubMed] [Google Scholar]
- 38.Nuanualsuwan S, Cliver DO. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl Environ Microbiol 2003;69:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griego SD, Weston CB, Adams JL, Tal-Singer R, Dillon SB. Role of p38 mitogen–activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol 2000;165:5211–5220. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Yamaya M, Sekizawa K, Hosoda M, Yamada N, Ishizuka S, Nakayama K, Yanai M, Numazaki Y, Sasaki H. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am J Physiol Lung Cell Mol Physiol 2001;280:L1115–L1127. [DOI] [PubMed] [Google Scholar]
- 41.Kaul P, Biagioli MC, Singh I, Turner RB. Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J Infect Dis 2000;181:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunstein MM, Hakonarson H, Whelan R, Yu Z, Grunstein JS, Chuang S. Rhinovirus elicits proasthmatic changes in airway responsiveness independently of viral infection. J Allergy Clin Immunol 2001;108:997–1004. [DOI] [PubMed] [Google Scholar]
- 43.Hussain MM. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front Biosci 2001;6:D417–D428. [DOI] [PubMed] [Google Scholar]
- 44.Laffont I, Takahashi M, Shibukawa Y, Honke K, Shuvaev VV, Siest G, Visvikis S, Taniguchi N. Apolipoprotein E activates Akt pathway in neuro-2a in an isoform-specific manner. Biochem Biophys Res Commun 2002;292:83–87. [DOI] [PubMed] [Google Scholar]
- 45.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation 2004;109:1022–1028. [DOI] [PubMed] [Google Scholar]
- 46.Papi A, Papadopoulos NG, Stanciu LA, Bellettato CM, Pinamonti S, Degitz K, Holgate ST, Johnston SL. Reducing agents inhibit rhinovirus-induced up-regulation of the rhinovirus receptor intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells. FASEB J 2002;16:1934–1936. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Hamati E, Lee P-K, Lee W-M, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol 2006;34:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.