Abstract
Rationale: The intermittent hypoxia (IH) that characterizes sleep-disordered breathing impairs spatial learning and increases NADPH oxidase activity and oxidative stress in rodents. We hypothesized that green tea catechin polyphenols (GTPs) may attenuate IH-induced neurobehavioral deficits by reducing IH-induced NADPH oxidase expression, lipid peroxidation, and inflammation.
Objectives: To assess the effects of GTP administered in drinking water on the cognitive, inflammatory, and oxidative responses to long-term (>14 d) IH during sleep in male Sprague-Dawley rats.
Methods: Cognitive assessments were conducted in the Morris water maze. We measured levels and expression of malondialdehyde (MDA), prostaglandin E2, p47phox subunit of NADPH oxidase, receptor for advanced glycation end products (RAGE), and glial fibrillary acidic protein expression in rodent brain tissue.
Measurements and Main Results: GTP treatment prevented IH-induced decreases in spatial bias for the hidden platform during the Morris water maze probe trails as well as IH-induced increases in p47phox expression within the hippocampal CA1 region. In untreated animals, IH exposure was associated with doubling of cortical MDA levels in comparison to room air control animals, and GTP-treated animals exposed to IH showed a 40% reduction in MDA levels. Increases in brain RAGE and glial fibrillary acidic protein expression were observed in IH-exposed animals, and these increases were attenuated in animals treated with GTP.
Conclusions: Oral GTP attenuates IH-induced spatial learning deficits and mitigates IH-induced oxidative stress through multiple beneficial effects on oxidant pathways. Because oxidative processes underlie neurocognitive deficits associated with IH, the potential therapeutic role of GTP in sleep-disordered breathing deserves further exploration.
Keywords: sleep apnea, cognition, inflammation, oxidative stress, hypoxia
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Sleep apnea is associated with neurocognitive deficits that are mediated at least in part by increased oxidative stress. It remains unclear how lifestyle issues such as dietary habits modify the susceptibility to the disease.
What This Study Adds to the Field
Oral supplements of green tea–derived polyphenols reduces the neural susceptibility to intermittent hypoxia during sleep in rodents.
In the last two decades, our awareness of sleep-disordered breathing (SDB) as a significant health problem that affects individuals throughout the lifespan has substantially increased (1). Obstructive sleep apnea (OSA), the most severe form of SDB, is characterized by repeated episodes of upper airway obstruction during sleep that induce intermittent hypoxia (IH) and sleep fragmentation. Left untreated, OSA may affect multiple target organs, ultimately leading to substantial metabolic, cardiovascular, and neurocognitive morbidities (2–4). In addition, patients suffering from OSA have been reported to have increased circulating markers of oxidative stress and inflammation and develop regional gray and white matter losses (5–7).
Chronic exposure to IH in rodents, modeling the hypoxia/reoxygenation patterns observed in severe sleep apnea patients, replicates many of the features of OSA in humans, such as increased oxidative stress, inflammatory signaling, cognitive deficits, and neurodegenerative and architectural changes within brain regions involved in learning and memory (8, 9).
Green tea contains a number of biologically active compounds which include epicatechin, epigallatocatechin, epicatechin gallate, and epigallatocatechin gallate (EGCG). These compounds, collectively referred to as green tea catechins polyphenols (GTPs), exhibit antioxidant properties as free radical scavengers, and recent epidemiological studies have shown that GTP compounds may reduce the risk of a variety of different diseases (10). Although the specific mechanisms of these protective effects remain unresolved, these findings have sparked intense interest in the potential therapeutic value of theses compounds in conditions associated with increased oxidative load (11–14). Therefore, the present study was undertaken to investigate the impact of oral GTP on oxidative stress, inflammatory load, and neurobehavioral impairments in our animal model of SDB.
A portion of the results from this study were previously reported in abstract form (15, 16).
METHODS
Animals
Young adult male Sprague-Dawley rats (250–300 g; n = 106) were used in this study (Charles River, Portage, MI) (see online supplement for animal husbandry details).
GTP
Commercially available Polyphenon 60 (P-60; Sigma-Aldrich, St. Louis, MO), which contains a minimum of 60% total catechins from green tea extract was administered in a concentration of 0.05% in drinking water for 3 days prior and throughout the intermittent hypoxia exposures. All drinking solutions were prepared fresh daily (see online supplement for details).
Body Weight and Fluid Intake
Male Sprague-Dawley Rats were randomly divided into experimental groups, half of which were exposed to IH, the other half to room air (RA). GTP was administered orally as previously outlined, with control groups receiving water alone. There was no difference in body weight or weight gain in the different groups. Fluid intake was measured every second day, and no significant differences in intake were found between the different groups, with all groups consuming approximately 10 ml per day on average.
IH Exposures
As previously described (8), rats were housed in identical, commercially designed chambers (75 × 50 × 50 cm) (Oxycycler model A44X0; Biospheryx, Redfield, NY) that were operated under a 12-hour light–dark cycle. The O2 concentration was continuously measured by an O2 analyzer, and was regulated throughout the 12-hour light cycle (06:00 a.m. to 06:00 p.m.) to achieve alternating intervals of 90 seconds 10% O2 with 90 seconds room air. Total air-exchange intervals during these experiments were approximately 240 seconds (for 10% O2) and 120 seconds (for RA), resulting in 10 hypoxic events per hour of exposure. For the remaining 12 hours of nighttime, oxygen concentration was kept at 21% (see online data supplement for details).
Behavioral Testing with the Morris Water Maze
Spatial place learning was assessed in a Morris water maze, as previously described (17, 18). Probe trials were conducted after the completion of place training and used as an indicator of spatial bias (see online supplement for details).
Lipid Peroxidation Assay
A spectrophotometric assay for malondialdehyde (MDA), a commonly used indicator of lipid peroxidation (MDA-586 kit no. 21044; OxisResearch, Portland, OR) was used in accordance with manufacturer's instructions to measure the relative MDA concentrations in rat brain cortex, prefrontal cortex, and hippocampus (see online supplement for details).
Prostaglandin E2 Assay
Cortical and hippocampal tissue concentrations of prostaglandin E2 (PGE2) were determined using a commercially available enzyme immunoassay kit (Oxford Biomedical Research, Oxford, MI) (see online supplement for details).
P47phox Quantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction
Quantititative real-time polymerase chain reaction (PCR) was performed with gene-specific primers for P47phox total RNA in rat brain tissue samples (see online supplement for details).
Immunohistochemistry
Double label immunolocalization was performed for glial fibrillary acidic protein (GFAP) (1:3,000) and Nissl (1:300) reactivity in rat brain cortex (n = 3; see online supplement for details).
Western Blotting
Brain tissue was rapidly harvested under pentobarbital anesthesia, and protein from CA1 hippocampal samples was extracted as previously described Membranes were incubated with either horseradish peroxidase linked anti-rat and anti-mouse antibodies (for β-actin and RAGE [receptor for advanced glycation end products], all 1:10,000; from Cell Signaling [Danvers, MA] and Jackson Labs [Bar Harbor, ME], respectively). Proteins were visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ). The intensities of the bands corresponding to the protein of interest were quantified using scanning densitometry, and the ratio of RAGE/β-actin was determined and used to compare groups (see online supplement for details).
Statistical Data Analysis
Untransformed (raw) and normalized data were analyzed with the SPSS statistical software package (SPSS, Inc., Chicago, IL). After the initial one- or two-way analysis of variance, the data were analyzed using Fisher's LSD (least significant difference) or Student-Newman-Keuls post hoc tests. A P value less than 0.05 was considered statistically significant.
RESULTS
Lipid Peroxidation: MDA Levels in Rat Cortex
Animals were exposed to IH or control conditions for 14 days before and during the 7 days of behavior assessments (21 total IH-exposure days), after which brain tissue was removed and processed for assessment of lipid peroxidation as indicated by MDA tissue levels. Figure 1 shows the average MDA concentrations in homogenates of cerebral cortex from IH and RA animals receiving water or GTP. A significant increase in MDA levels was observed in IH-water–treated animals in comparison to RA-water–, RA-GTP–, and IH-GTP–treated animals (n = 6 per group; P < 0.05). The water-IH group showed a doubling of cortical MDA levels compared with room air, whereas GTP-IH animals showed 33% reduction in MDA levels compared with water-IH animals. Animals exposed to GTP-RA showed similar reductions in MDA levels compared with water-RA.
Figure 1.
Relative malondialdehyde (MDA) production, an indicator of lipid peroxidation, in cerebral cortex of rats exposed to room air (RA) or intermittent hypoxia (IH) and treated with green tea polyphenols (GTP) or vehicle (n = 6 per group; *P < 0.05, #P < 0.05 vs. RA). W = water.
NADPH (p47phox Reverse Transcriptase–PCR)
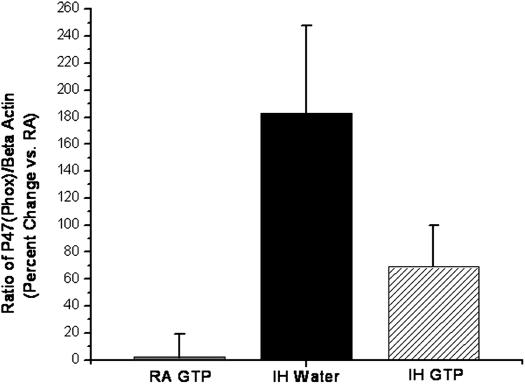
Gene expression of the p47phox subunit of NADPH oxidase, the major enzyme underlying oxygen radical production, was measured in cortical brain regions using quantitative reverse transcriptase (RT)–PCR. Cortical levels of p47phox mRNA (normalized to RA-water control animals) were significantly elevated in IH-water–treated animals in comparison to RA-GTP– and IH-GTP–treated animals (n = 6 per group; P < 0.05) (Figure 2). We observed a 183% elevation in the ratio of p47phox to β-actin mRNA in water-IH animals as compared with RA-water and RA-GTP (P < 0.003). This elevation was significantly attenuated in IH-GTP–treated animals (P < 0.01).
Figure 2.
Mean ratio of p47phox to β-actin mRNA as an indicator of NADPH oxidase expression in the cerebral cortex of rats exposed to room air (RA) or intermittent hypoxia (IH) and treated with green tea polyphenols (GTP) or vehicle expressed as percentage change relative to control RA water (n = 6 per group; *P < 0.05).
Inflammation (PGE2)
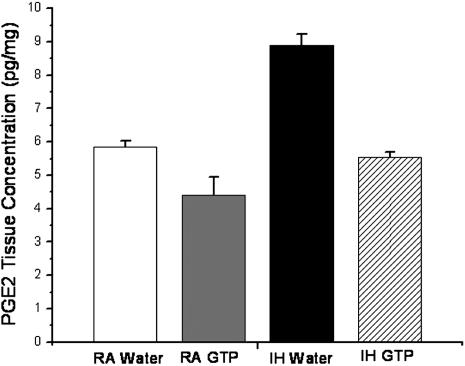
To determine whether GTP can influence COX-2–mediated increases in inflammatory signaling within the hippocampus after exposure to IH, PGE2 concentrations were measured using ELISA. Concentrations of PGE2 were significantly increased within the hippocampal CA1 region by IH-water–treated animals compared with RA control animals (P < 0.05; n = 3) (Figure 3). PGE2 levels were significantly attenuated in animals receiving GTP during IH, such that no significant differences emerged in RA-water–, RA-GTP–, and IH-GTP–treated animals.
Figure 3.
Relative prostaglandin E2 (PGE2) production in the hippocampal CA1 region of rats exposed to room air (RA) or intermittent hypoxia (IH) and treated with green tea polyphenols (GTP) or vehicle (n = 3 per group; *P < 0.05, #P < 0.05 vs. RA).
Behavior Testing (Morris Water Maze)
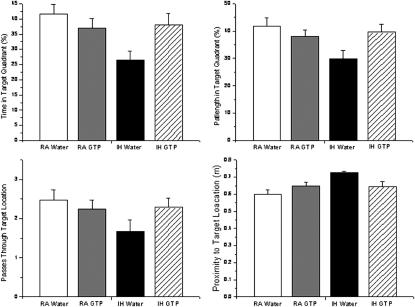
The results of the behavior testing are summarized in Figure 4. Upon completion on the final probe trial-test, in which the platform was removed to test spatial bias, IH-GTP–treated rats displayed a significantly greater spatial bias for the target platform in comparison to IH-water–treated animals, as illustrated by the percentage of time spent in quadrant, the path length in the target quadrant, the numbers of passes over the previous target platform, and the mean proximity to the previous target platform (P < 0.05 for all measures; n = 19–25 per group). Post hoc analyses revealed no significant differences between IH-GTP–, RA-GTP–, and RA-water–treated animals. No significant group differences were observed on latency and path length to locate a simple, visible platform task (data not shown), indicating that no overt sensorimotor disturbances are observed in our model.
Figure 4.
Summary of probe trial performance after completion of water maze testing in rats exposed to room air (RA) or intermittent hypoxia (IH) and treated with green tea polyphenols (GTP) or vehicle (n = 19–25 per group; *P < 0.05).
RAGE
RAGE protein expression was determined in hippocampal CA1 lysates via Western blotting. The ratio of RAGE/β-actin within the hippocampal CA1 region was elevated in IH-water–treated animals in comparison to RA control animals (P < 0.01; n = 4) (Figure 5). This elevation of RAGE was significantly attenuated in animals receiving GTP during IH, such that no significant differences emerged in RA-water–, RA-GTP–, and IH-GTP–treated animals.
Figure 5.
Upper panels: Representative Western blots for receptor for advanced glycation end products (RAGE) and β-actin in CA1 hippocampal lysates from rats exposed to room air (RA) or intermittent hypoxia (IH) and treated with green tea polyphenols (GTP) or vehicle. Lower panel: Mean ratios of RAGE to β-actin in CA1 hippocampal lysates from rats exposed to RA or IH and treated with GTP or vehicle (n = 4 per group; *P < 0.01).
Immunohistochemistry (GFAP)
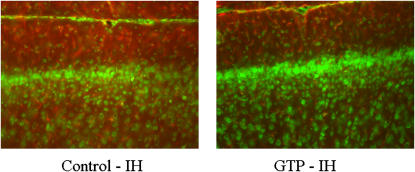
Previously, we have demonstrated that increased glial proliferation occurs in the cortex of rats exposed to IH (8). To determine whether GTPs can influence glial proliferation after IH exposure, immunohistochemistry was performed for the astrocyte marker GFAP. GTP administration reduced expression of GFAP in the cortex, indicating that polyphenolic compounds are capable of attenuating IH-induced increases in cortical glial proliferation (Figure 6).
Figure 6.
Coronal cortical sections illustrating glial fibrillary acidic protein (red) and Nissl (green) immunoreactivity in rats exposed to intermittent hypoxia (IH) and treated with vehicle (left panel) or green tea polyphenols (GTP) (right panel).
DISCUSSION
During the last two decades, OSA has increasingly been recognized as a serious and frequent health condition with potential long-term morbidities that include learning and psychological disabilities, metabolic consequences, and increased severity and prevalence of cardiovascular disorders (2, 4, 19–21).
Work from our laboratory revealed that the hippocampus and prefrontal cortex, critical structures for learning and memory, are particularly sensitive to the hypoxic events occurring during extended periods of episodic hypoxia during sleep and that these changes lead to significant cognitive deficits in the rodent (8, 17, 22, 23). In addition, a growing body of evidence suggests that the adverse neurobehavioral consequences imposed by IH stem, at least in part, from activation of oxidative stress and inflammatory signaling cascades. Up-regulation of genes that play important roles in the induction and maintenance of inflammatory processes, such as cytokines and cyclooxygenases (COX), as well as increased eicosanoid and platelet-activating factor (PAF)-mediated neuroinflammation have been demonstrated after exposure to IH in rodents (9, 18, 24–27). Such changes can amplify nitric oxide-induced cellular injury via oxidative and nitrosative pathways, most likely through nuclear factor-κB–dependent pathways (28, 29). The collective action of such oxidative and inflammatory processes likely compromises cell viability within sensitive brain regions while failing to appropriately recruit inducible defense processes, resulting in increased neuronal vulnerability and functional deficits. Although the causal relationship between inflammatory and oxidative pathways remains to be elucidated, the available data suggest that reductions in oxidative stress may offer great potential therapeutic utility in animal models of sleep apnea and in patients suffering from the disease (9, 18, 24–27).
Polyphenolic compounds are present in different food items, such as tea, wine, fruits, and vegetables, and their antioxidative capacities have been extensively studied in the past 15 years (30). Green tea, one of the world's most widely consumed beverages, contains a number of these biologically active polyphenolic compounds, suggesting that the active components of green tea may be beneficial and neuroprotective in conditions of increased oxidative load. Recent studies have demonstrated the neuroprotective activity of GTP in animal models of neurodegenerative conditions such as Parkinson's and Alzheimer's disease and their ability to markedly reduce hypoxic-ischemic tissue loss in models of ischemic stroke through inhibition of caspase-3 activation and proteolytic cleavage of its substrates (31–34). Although the potential mechanisms whereby GTP may prevent neurological deficits remain to be fully elucidated, recent evidence suggests that GTP may operate as potent free oxygen radical scavengers and protect the brain and other organs from lipid peroxidation injury (35). For example, EGCG has been shown to reducing H2O2 and ferrous ion-induced lipid peroxidation in gerbil brain homogenates and to inhibit lipid peroxidation in kidney and liver tissue after induction of oxidative stress with bromotrichloromethane (36, 37). These compounds easily reach brain tissue after oral ingestion to initiate and potentially reduce oxidative stress and inflammation, making them extremely attractive therapeutic compounds, given the lack of a neuronal morphophysiological barrier to GTP access (38, 39). The findings presented in this study provide the first indication that oral GTP administration attenuates not only the oxidative and inflammatory mechanisms associated with IH during sleep but also prevents IH-induced spatial learning deficits in the rat.
Lipid Peroxidation (MDA Expression)
Oxidative stress is a well-established mechanism of cellular injury in the brain because brain tissue contains a large amount of polysaturated fatty acids, which are highly susceptible to oxidative reactions. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds. These include reactive carbonyl compounds, of which the most abundant is MDA, a commonly used indicator of lipid peroxidation, oxidative stress, and subsequent cellular injury in cells and tissues. Consistent with our previous findings, exposure to IH was associated with a significant increase in lipid peroxidation in the cortex of animals exposed to IH (18, 40). In our experiments, we also observed that IH-induced increases in MDA were attenuated by GTP administration, and these findings are consistent with previous studies whereby EGCG (the most active GTP) seems to be neuroprotective against neuronal damage after global ischemia in gerbils (37) and reduces lipid peroxidation and is neuroprotective for hydrocephalus-induced periventricular oxidative damage in a rat model (35).
NADPH Oxidase (RT-PCR for mRNA Expression of p47phox)
NADPH-oxidase–dependent production of superoxide radical (O2−) has been identified as a major contributor to oxidative injury in the brain under conditions of severe hypoxia and inflammation (41–45). NADPH oxidase has also been implicated in neurodegeneration, such as Alzheimer's and Parkinson's disease, and NADPH oxidase is increasingly recognized for its role in health and disease, necessary for normal immunity and cell signaling (44). Long-term exposures to frequent hypoxia/reoxygenation events that mimic the altered oxygenation patterns of SDB have been shown to induce NADPH oxidase in selected brain regions, suggesting that activation of this enzyme may partly underlie the increased neuronal inflammation and oxidative stress observed in animal models of SDB (25). Our findings provide further support for the hypothesis that intermittent hypoxia increases NADPH oxidase subunit protein expression in hypoxia sensitive brain regions involved in learning and memory and that GTP administration was able to attenuate the increase in NADPH oxidase gene expression under IH conditions. These findings are consistent with reports that high doses of epigallocatechin were able to lessen neuronal NADPH expression in rats exposed to acute hypoxia (38).
Inflammatory Signaling (PGE2)
PGE2 is a major prostanoid produced by COX with diverse physiological effects, such as increased vascular permeability, vasodilatation, and induction of neutrophil chemotaxis, and is induced in response to various physiological and pathological stimuli, including ischemia and hypoxia (see Reference 46 for review). PGE2 is constitutively present in the rat brain, particularly in the cortex and hippocampus (47). As described in previous work from our laboratory, IH increases PGE2 tissue concentrations, indicating that up-regulation and activation of COX-2 in rat hippocampus and cortex plays a mechanistic role in IH-mediated neurobehavioral deficits in IH (27). Consistent with the putative antiinflammatory role of GTP, we found that the addition of GTP to the drinking water significantly attenuated IH-induced increases in hippocampal tissue PGE2 concentrations, suggesting that these compounds are capable of reducing inflammatory signaling in our rodent model of SDB. These effects are consistent with similar effects of green tea polyphenols in peripheral tissues, as shown by August and colleagues, who reported a decreased level of PGE2 in the rectal mucosa after consumption of green tea in humans (48).
Attenuation of IH-induced RAGE Expression
Advanced glycation end products (AGEs) resulting from nonenzymatic glycation of proteins and lipids accumulate during normal aging and at an accelerated rate in age-related disorders such as diabetes mellitus and atherosclerosis (49, 50). The interaction of AGEs with their main receptor, RAGE, induces intracellular generation of reactive oxygen species and up-regulation of inflammatory pathways dependent on RAGE signal transduction (51, 52). In turn, the increased oxidative stress and inflammation leads to increased expression of RAGE and ultimately increased AGE–RAGE ligand interactions, thus prompting a vicious cycle that is thought to contribute and amplify inflammatory/degenerative processes (52, 53). For example, treatment with soluble RAGE (sRAGE), the extracellular ligand binding domain of RAGE that binds ligands and blocks their interactions and activation of cell surface receptors, decreases inflammation and oxidant stress in a number of in vivo models (54).
A recent report by Tan and colleagues (55) indicates that AGE concentrations are elevated in patients with obstructive sleep apnea, thus promoting increased AGE–RAGE ligand interactions and propagation of oxidative stress through stimulation of NADPH oxidase. In the present study, IH exposure was associated with significant elevations in the expression of the RAGE receptor in IH-sensitive brain regions, such as the CA1, which were abrogated by concurrent administration of GTP in the drinking water. This is consistent with reports that GTPs are capable of reducing the activity of compounds leading to the formulation of AGEs (56), although it is unclear whether the observed reduction in our study is due to inhibition of AGE formation or to decreases in NADPH oxidase-mediated changes in gene expression (52).
Enhanced Glial Proliferation
Previously, we have demonstrated that enhanced glial proliferation occurs after IH exposure (8). Pathological activation of microglia is associated with various neurodegenerative diseases, and enhanced glial proliferation has been postulated to participate in the activation of positive feedback loops, which initiate ascending spirals of cellular activation and dysfunction in such pathophysiological states, primarily due to subsequent loss of buffering functions (57, 58). Zhan and colleagues (25) have postulated that increased activation of NADPH oxidase underlies subsequent IH-induced carbonylation of proteins, which activate microglial proinflammatory responses and promote such a positive feedback loop. Consistent with this hypothesis, GTP administration not only reduced IH-induced increased in NADPH oxidase gene expression but also attenuated the increases in GFAP expression in the rat cortex. Although these results suggest that the glial proliferation observed in our model is a reactive response, by-products of oxidative stress, such as lipid peroxidation and carbonylation products, also modulate cell proliferation and differentiation and thereby may exert local and systemic effects that may be both harmful and beneficial (59).
Attenuation of IH-induced Spatial Learning Deficits
Exposure to prolonged periods of IH, such as occurs in patients with sleep apnea, is associated with marked impairments of hippocampus-dependent learning tasks (e.g., spatial, reference version of the Morris water maze). These deficits are attenuated by reductions in oxidative stress and inflammatory signaling cascades through pharmacological interventions (18, 27). Similarly, on probe trials administered after completion Morris water maze training, GTP-treated rats exposed to IH displayed significantly greater spatial bias for the previous hidden platform position, indicating that GTPs are capable of attenuating IH-induced spatial learning deficits, presumably through reductions of the degenerative oxidative and inflammatory mechanisms associated with IH, and may represent a potential interventional strategy for patients with SDB.
Conclusions
In summary, we report novel findings on the effect of GTPs in the context of intermittent hypoxia during sleep, a condition with markedly important clinical relevance, considering the high prevalence of SDB. We herein show that GTP administration produces significant reductions in the induction of pathophysiological mechanisms implicated in the neuronal damage associated with IH and that parallel improvements in IH-induced hippocampus-dependent deficits occur with GTP intake. Although the rodent model simulates the oxygenation pattern of moderate to severe SDB, it does not incorporate other potentially injurious elements of SDB, such as sleep fragmentation or recurring hypercapnia. Notwithstanding such considerations, our present findings indicate that addition of a combination of various polyphenols with substantial antioxidant properties to the drinking water will attenuate oxidative stress and inflammatory mechanisms underlying IH-induced end-organ damage in the rat brain. These novel findings clearly warrant further research aimed at defining the roles of GTP compounds as potential pharmacologic supplements in the prevention of SDB-induced neurocognitive morbidities.
Supplementary Material
Supported by National Institutes of Health grants HL-69932 and 2P50 HL60296 and a research fellowship from the Swiss National Foundation to I.C.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200701-110OC on February 14, 2008
Conflict of Interest Statement: I.C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G. is on the national speaker bureau of Merck Company. E.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.C.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.W.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mitchell RB. Sleep-disordered breathing in children: are we underestimating the problem? Eur Respir J 2005;25:216–217. [DOI] [PubMed] [Google Scholar]
- 2.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep 2000;23:369–381. [PubMed] [Google Scholar]
- 3.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005;99:1998–2007. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev 2005;9:211–224. [DOI] [PubMed] [Google Scholar]
- 5.Alchanatis M, Deligiorgis N, Zias N, Amfilochiou A, Gotsis E, Karakatsani A, Papadimitriou A. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J 2004;24:980–986. [DOI] [PubMed] [Google Scholar]
- 6.Lavie L. Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev 2003;7:35–51. [DOI] [PubMed] [Google Scholar]
- 7.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:1382–1387. [DOI] [PubMed] [Google Scholar]
- 8.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 2001;21:2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004;27:194–201. [DOI] [PubMed] [Google Scholar]
- 10.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr 2004;134(12, Suppl):3431S–3440S. [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Guo Q, Xin W. Free radical scavenging by green tea polyphenols. Methods Enzymol 2001;335:217–231. [DOI] [PubMed] [Google Scholar]
- 12.Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta 1996;1304:210–222. [DOI] [PubMed] [Google Scholar]
- 13.Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean ME. Tea flavonoids and cardiovascular health. QJM 2001;94:277–282. [DOI] [PubMed] [Google Scholar]
- 14.Oldreive C, Zhao K, Paganga G, Halliwell B, Rice-Evans C. Inhibition of nitrous acid-dependent tyrosine nitration and DNA base deamination by flavonoids and other phenolic compounds. Chem Res Toxicol 1998;11:1574–1579. [DOI] [PubMed] [Google Scholar]
- 15.Burckhardt IC, Cheng Y, Row BW, Gozal D. Green tea catechin polyphenols reduce susceptibility to intermittent hypoxia (IH)-induced spatial learning deficits in the rat. Neuroscience 2005, the 35th Annual Meeting of the Society for Neuroscience, Washington, DC; 2005.
- 16.Burckhardt IC, Cheng Y, Row BW, Gozal D. Oral green tea catechin polyphenols attenuate learning deficits and oxidative stress in rat brain following intermittent hypoxia during sleep. Sleep 2006, the 20th Annual Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT; 2006.
- 17.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 2002;52:449–453. [DOI] [PubMed] [Google Scholar]
- 18.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 2003;167:1548–1553. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O'Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiolo Neurobiol 2003;136:167–178. [DOI] [PubMed] [Google Scholar]
- 20.Redline S,Young T. Epidemiology and natural history of obstructive sleep apnea. Ear Nose Throat J 1993;72:20–21, 24–26. [PubMed] [Google Scholar]
- 21.Anstead M, Phillips B. The spectrum of sleep-disordered breathing. Respir Care Clin N Am 1999;5:363–377. [PubMed] [Google Scholar]
- 22.Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, Sachleben LR Jr, Guo SZ. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. Eur J Neurosci 2003;18:2335–2342. [DOI] [PubMed] [Google Scholar]
- 23.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett 2001;305:197–201. [DOI] [PubMed] [Google Scholar]
- 24.Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med 2005;171:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 2005;172:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 2004;17:44–53. [DOI] [PubMed] [Google Scholar]
- 27.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 2003;168:469–475. [DOI] [PubMed] [Google Scholar]
- 28.Eu JP, Liu L, Zeng M, Stamler JS. An apoptotic model for nitrosative stress. Biochemistry 2000;39:1040–1047. [DOI] [PubMed] [Google Scholar]
- 29.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem 2007;101:577–599. [DOI] [PubMed] [Google Scholar]
- 30.Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 2005;16:577–586. [DOI] [PubMed] [Google Scholar]
- 31.Hong JT, Ryu SR, Kim HJ, Lee JK, Lee SH, Kim DB, Yun YP, Ryu JH, Lee BM, Kim PY. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res Bull 2000;53:743–749. [DOI] [PubMed] [Google Scholar]
- 32.Frank B, Gupta S. A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry 2005;17:269–286. [DOI] [PubMed] [Google Scholar]
- 33.Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem 2004;15:506–516. [DOI] [PubMed] [Google Scholar]
- 34.Jeong JH, Kim HJ, Lee TJ, Kim MK, Park ES, Choi BS. Epigallocatechin 3-gallate attenuates neuronal damage induced by 3-hydroxykynurenine. Toxicology 2004;195:53–60. [DOI] [PubMed] [Google Scholar]
- 35.Etus V, Altug T, Belce A, Ceylan S. Green tea polyphenol (-)-epigallocatechin gallate prevents oxidative damage on periventricular white matter of infantile rats with hydrocephalus. Tohoku J Exp Med 2003;200:203–209. [DOI] [PubMed] [Google Scholar]
- 36.Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, Oguni I, Konomoto H. Effect of tea (Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull 1995;18:1006–1008. [DOI] [PubMed] [Google Scholar]
- 37.Lee SR, Im KJ, Suh SI, Jung JG. Protective effect of green tea polyphenol (-)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother Res 2003;17:206–209. [DOI] [PubMed] [Google Scholar]
- 38.Wei IH, Wu YC, Wen CY, Shieh JY. Green tea polyphenol (-)-epigallocatechin gallate attenuates the neuronal NADPH-d/nNOS expression in the nodose ganglion of acute hypoxic rats. Brain Res 2004;999:73–80. [DOI] [PubMed] [Google Scholar]
- 39.Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 2002;33:1693–1702. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004;126:313–323. [DOI] [PubMed] [Google Scholar]
- 41.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med 2006;40:376–387. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res 2006;1090:182–189. [DOI] [PubMed] [Google Scholar]
- 43.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 2003;28:502–508. [DOI] [PubMed] [Google Scholar]
- 44.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci 2005;25:9176–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Therade-Matharan S, Laemmel E, Duranteau J, Vicaut E. Reoxygenation after hypoxia and glucose depletion causes reactive oxygen species production by mitochondria in HUVEC. Am J Physiol Regul Integr Comp Physiol 2004;287:R1037–R1043. [DOI] [PubMed] [Google Scholar]
- 46.Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Brain Res Rev 2006;52:201–243. [DOI] [PubMed] [Google Scholar]
- 47.Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 1995;355:296–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.August DA, Landau J, Caputo D, Hong J, Lee MJ, Yang CS. Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev 1999;8:709–713. [PubMed] [Google Scholar]
- 49.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation 2006;113:1226–1234. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 2000;1498:99–111. [DOI] [PubMed] [Google Scholar]
- 51.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 2002;105:816–822. [DOI] [PubMed] [Google Scholar]
- 52.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–E694. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem 2000;275:25781–25790. [DOI] [PubMed] [Google Scholar]
- 54.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005;15:16R–28R. [DOI] [PubMed] [Google Scholar]
- 55.Tan KC, Chow WS, Lam JC, Lam B, Bucala R, Betteridge J, Ip MS. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep 2006;29:329–333. [DOI] [PubMed] [Google Scholar]
- 56.Lo CY, Li S, Tan D, Pan MH, Sang S, Ho CT. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol Nutr Food Res 2006;50:1118–1128. [DOI] [PubMed] [Google Scholar]
- 57.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol 2001;171:29–45. [DOI] [PubMed] [Google Scholar]
- 58.Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 2001;17:436–443. [DOI] [PubMed] [Google Scholar]
- 59.Zarkovic N, Zarkovic K, Schaur RJ, Stolc S, Schlag G, Redl H, Waeg G, Borovic S, Loncaric I, Juric G, et al. 4-Hydroxynonenal as a second messenger of free radicals and growth modifying factor. Life Sci 1999;65:1901–1904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.