Abstract
Background
Since its first detection, characterization of R. felis has been a matter of debate, mostly due to the contamination of an initial R. felis culture by R. typhi. However, the first stable culture of R. felis allowed its precise phenotypic and genotypic characterization, and demonstrated that this species belonged to the spotted fever group rickettsiae. Later, its genome sequence revealed the presence of two forms of the same plasmid, physically confirmed by biological data. In a recent article, Gillespie et al. (PLoS One. 2007;2(3):e266.) used a bioinformatic approach to refute the presence of the second plasmid form, and proposed the creation of a specific phylogenetic group for R. felis.
Methodology/Principal Findings
In the present report, we, and five independent international laboratories confirmed unambiguously by PCR the presence of two plasmid forms in R. felis strain URRWXCal2 T, but observed that the plasmid content of this species, from none to 2 plasmid forms, may depend on the culture passage history of the studied strain. We also demonstrated that R. felis does not cultivate in Vero cells at 37°C but generates plaques at 30°C. Finally, using a phylogenetic study based on 667 concatenated core genes, we demonstrated the position of R. felis within the spotted fever group.
Significance
We demonstrated that R. felis, which unambiguously belongs to the spotted fever group rickettsiae, may contain up to two plasmid forms but this plasmid content is unstable.
Introduction
Rickettsia felis (R. felis) was first detected in 1990 in American Ctenocephalides felis fleas using electron microscopy, and named the ELB agent after the Elward laboratory (Soquel, CA) where the flea colony was raised [1]. It was later detected by PCR in humans with a murine typhus-like illness [2]–[6]. Unfortunately, despite a first phylogenetic study clearly showing its classification within the spotted fever group (SFG) [7], confusion was brought by further reports, which attributed to the ELB agent, renamed R. felis, several characteristics of R. typhi [8]–[11]. It was later demonstrated that these data, including the protein profile [11], antigenic properties, growth conditions [8], [9], and antibiotic susceptibility [10], resulted from the contamination of a R. felis culture with R. typhi [12]. As a matter of fact, fleas can be infected by both R. typhi and R. felis [13], which may thus have been the source of contamination. In 2001, R. felis was cultivated from cat fleas at low temperature (28°C), and was established for the first time [2]. It was then deposited as strain URRWXCal2 T in two official collections: the American Type Culture Collection (ATCC VR-1525), and the Collection de Souches de l'Unité des Rickettsies (CSUR R121). Subsequently, another two teams were able to successfully grow R. felis, also at temperatures ≤32°C [14], [15] and not at 35–37°C as initially reported [9], [10], [12]. Phenotypic characterization of strain URRWXCal2 T demonstrated that its antibiotic susceptibility [16] and antigenic properties [17] classified it within the SFG. In 2005, we sequenced the genome of R. felis strain URRWXCal2 T [18] and demonstrated, using bioinformatics, pulsed field gel electrophoresis and southern blot, that this strain had two forms of the same plasmid, i. e., a large, (pRF, 62-kb), and a small (pRFδ, 39-kb) forms. The pRF and PRFδ plasmids had identical sequences with the exception of an additional 24 ORFs in pRF. Then, we confirmed these results by PCR assays specifically targeting each plasmid [18]. The two plasmids were also detected in a collection of Ctenocephalides felis fleas from various locations. However, in a recent bioinformatic analysis of the R. felis genome sequence, Gillespie et al. questioned our results and proposed that the pRFδ plasmid was an artefact from genome assembly [19]. These authors based in part their conclusion on the results from Pornwiroon et al. who failed to detect the pRFδ plasmid in R. felis strain LSU [14], a strain cultivated in C. felis fleas at Louisiana State University [20]. In addition, on the basis of the analysis of a few genes, they proposed the classification of R. felis in a fourth phylogenetic lineage within the Rickettsia genus [19].
As our previous work had been carefully performed and experimentally confirmed, we believe that the conclusions of Gillespie et al. were not appropriate. Therefore, we asked five independent laboratories worldwide to check the presence of two plasmids in R. felis. We also evaluated the presence of both plasmids in various specimens and in cloned R. felis. Finally, we conducted a phylogenetic study based on 667 concatenated Rickettsia core protein-encoding genes, and tested new specimens.
Results
Presence of two plasmids in R. felis strain URRWXCal2 T and variation of plasmid content according to the passage history
Using all four primer pairs, we obtained PCR products of the expected sizes from R. felis cultivated from the initial isolate [2]. Non-template controls were negative. The sequence obtained from the pRFa-pRFd amplicon was identical to GenBank accession number NC_007111, whereas pRFa-pRFb and pRFc-pRFd amplicons were identical to accession number NC_007110. Thus, we could reproduce our previously reported result and confirmed the presence of the two plasmids in R. felis strain URRWXCal2 T. However, in order to obtain indisputable results, we proposed five independent laboratories worldwide to perform these PCR assays. To avoid any interpretation bias, we provided these laboratories with anonymized kits. All five laboratories obtained similar PCR results (Figure 1a, Table 1). A PCR product of the expected size was obtained for all 4 assays from DNA specimen 1, whereas for DNA specimens 2 and 3, only the AF-AR PCR provided a positive amplification. Negative controls were negative for all assays. Each of the five laboratories obtained a B-E PCR nucleotide sequence for R. felis identical to GenBank accession number NC_007111. Sequences were deposited in GenBank under accession numbers EU155007, EU056172, EU022170, EU040362, and EU017504 for laboratories L1 to L5, respectively. Further, when attempting to clone R. felis cells with a 40 cell culture passage history at various temperatures using the plaque assay, we obtained lysis plaques at 30°C but no culture at 37°C (Figure 2). Of 20 clones grown from single R. felis cells and individually collected, we obtained PCR products of the expected sizes for the two plasmid forms in 15 clones (75 %) but only the large plasmid form in the remaining five clones. In addition, only the small plasmid form was detected from the positive control DNA extracted from R. felis with a 50 cell culture passage history. These data demonstrated that the plasmid content of R. felis may vary according to culture history, with a small plasmid form being unstable. R. felis may exhibit from two (R. felis grown from initial frozen culture) to one plasmid form (R. felis with a 50 cell culture passages). Such a phenomenon was recently reported by Baldridge et al. who demonstrated that R. peacockii lost its plasmids during serial passage in cell culture [21].
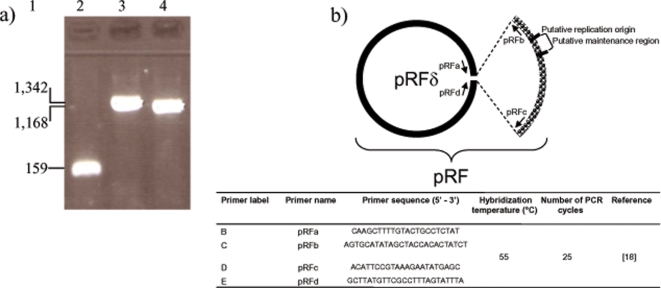
Figure 1. The R. felis plasmids.
Specific PCR amplification of the two R. felis plasmid forms. Lane 1: Molecular size (bp); lane 2: pRFa-pRFb amplicon; lane 3: pRFc-pRFd amplicon; lane 4: pRFa-pRFd amplicon; b) Schematic representation of R. felis plasmids indicating the position of PCR primers used to amplify the pRF (pRFa/pRFb and pRFc/pRFd primer pairs) and pRFδ (pRFa/pRFd primer pair) plasmids.
Table 1. PCR results obtained by all five tester laboratories.
| DNA specimen (species) | PCR assay (primers) | |||
| B–E (pRFa-pRFd) | B-C (pRFa-pRFb) | D-E (pRFc-pRFd) | AF-AR | |
| DNA1 (R. felis) | + | + | + | + |
| DNA2 (R. conorii) | - | - | - | + |
| DNA3 (R. africae) | - | - | - | + |
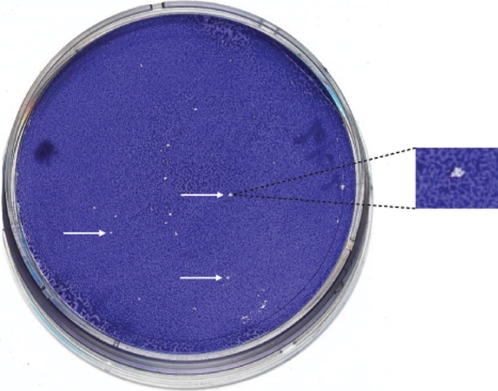
Figure 2. Cloning of R. felis cells using a plaque assay.
White arrows show individual lysis plaques. Right, a lysis plaque was enlarged.
In their article, Gillespie et al. speculated, based on an in silico analysis, that the small plasmid form detected in R. felis was an artefact of genome assembly [19]. In our previous study, we were surprised to find two plasmid forms in R. felis, but found them both by pulsed field gel electrophoresis (Figure 3), southern blot, and genome assembly [18]. Moreover, we verified biologically that the small plasmid form can specifically be amplified by PCR, showing that the 2 plasmid forms exist both in cell culture and in the wild by amplification of R. felis DNA from C. felis fleas [18]. Therefore, the results that we and five independent laboratories obtained in the present study unambiguously confirm that our previous data did not result from a bioinformatic error and that two plasmid forms may co-exist in R. felis (Figure 1b). Moreover, by demonstrating in gels that the two plasmid forms were present in almost equal quantity in culture, we speculated that both forms may be present in a single R. felis cell (Figure 3). To explore this hypothesis, we cloned single R. felis cells and demonstrated that the small plasmid form lacked in 25 % of individual cells of this species. Baldridge et al. recently reported the detection of plasmids in five Rickettsia species, including two, i.e., R. peacockii and R. amblyommii which had two plasmids [21]. These results, as well as ours, contradict the speculations of Gillespie et al. [19] that R. felis cannot have two plasmids.
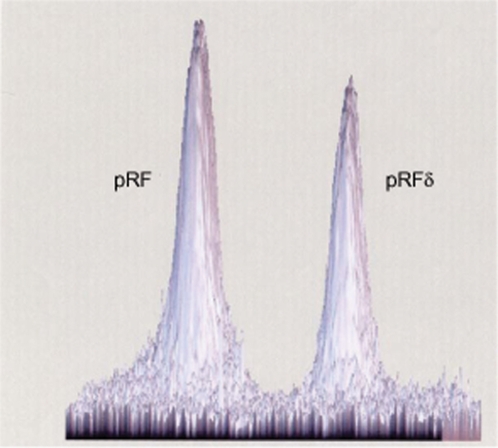
Figure 3. Determination of the R. felis plasmid ratio.
The Southern blot obtained by hybridizing R. felis genomic DNA digested with PvuI and resolved by PFGE with probes specific for each plasmid form [18] was digitalized by transmission scanning (ImageScanner, Amersham Biosciences). The quantification of each labelled plasmid band was estimated by analysis with the ImageMaster 2D Platinium Version 6.0 software (Amersham Biosciences). The pRF and pRFδ spots represented 57% and 43%, respectively, of the hybridization intensity.
Variation of plasmid content in R. felis according to the strain
In addition to their bioinformatics analysis, Gillespie et al. based their conclusions on the results published by Pornwiroon et al. who did not detect the pRFδ plasmid form from R. felis strain LSU [14]. In the present study, the expert laboratory L1 detected the pRF but not the pRFδ plasmid form in R. felis strain LSU DNA provided by these authors, thus confirming their data [14]. Subsequently, we tested another R. felis strain, the RF2125 strain endemic in Archaeopsylla erinacei fleas from Algeria [22]. Although the pRF plasmid form was detected from 64 A. erinacei, we failed to detect the pRFδ plasmid form in any of these. Thus, in addition to culture conditions, the plasmid content of R. felis may vary from one strain to another. The former hypothesis is supported by the fact that the URRWXCal2 T strain may have one or two plasmid forms as demonstrated by our results, that the LSU strain, belonging to the same genotype, has one plasmid form [14], and that the RF2125 strain, genetically different from the other two strains (Table 2), may have one or no plasmid.
Table 2. Genetic variability of R. felis strain URRWXCal2 T compared to other studied strains.
Phylogeny of R. felis
Using sequences from 15 chromosome-encoded proteins or from 21 conserved hypothetical proteins, Gillespie et al. proposed the creation of a fourth phylogenetic cluster within the Rickettsia genus, the “transitional group”, that contained R. felis [14]. However, the data produced by these authors do not provide any evidence that R. felis belongs to “a lineage distinct from other previously established taxonomic categories for rickettsiae”. As stated by the authors, their phylogenetic study is similar to other recently published rickettsial trees, which showed no evidence or need for the creation of a fourth lineage [23], [24]. This result even contradicts a previous article by the same team where R. felis was clearly associated with the SFG [25]. Initial speculations on an intermediate status of R. felis, with phenotypic properties similar to R. typhi but a genetic clustering with spotted fever group rickettsiae, resulted from studies that have not been reproduced [1], [8]–[11], [25], [26]. Moreover, the only strain recovered from this work was R. typhi and contamination was acknowledged in one of these four early papers [12]. By analysing ad hoc genes, one may also cluster R. felis and R. bellii because both species share unique characteristics among rickettsiae such as the largest chromosomes, or the presence of tra clusters and transposases [18], [23].
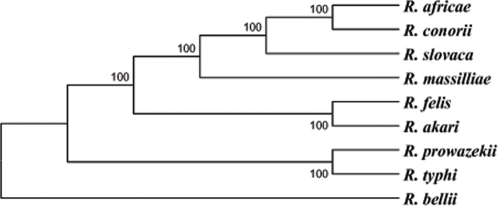
Moreover, the analysis of various genes that provided reliable phylogenetic organisations of rickettsiae demonstrated that R. felis was placed in a cluster also including R. akari and R. australis within the SFG [27]–[29]. As a matter of fact, R. australis is transmitted by ticks and has unambiguously been classified within the spotted fever group [30]. However, the reliability of phylogenetic studies based on selected genes may be impaired by a selection bias, in particular due to recombination or lateral gene transfer, as previously described for Rickettsia species [31]. In the most complete phylogenetic study of Rickettsia species performed to date, based on complete genome sequences, R. felis was clearly classified within the SFG [32]. Herein, with the same method as that used by Gillespie et al. [19], the phylogenetic tree that we inferred from the comparison of 667 concatenated core genes of Rickettsia unambiguously placed R. felis within the SFG (Figure 4). Therefore, our data do not demonstrate any necessity to create a new cluster. In addition, the distance between R. felis and other SFG species is not bigger than that between R. helvetica, another SFG species, and other SFG members for example [28].
Figure 4. Phylogenetic tree inferred from the comparison of 667 concatenated Rickettsia core protein-coding genes using the maximum parsimony method.
Bootstrap values are indicated at branch nodes. A similar topology was obtained using the Neighbor-Joining analysis method.
Correlation host vector-phylotype
The hypothesis that Rickettsia species have acquired virulence after the divergence of R. bellii and R. canadensis is misleading [19]. By omitting some data, one may build a simplified model of host-vector-rickettsia co-speciation, with R. prowazekii being associated with lice, R. felis with fleas and R. rickettsii with ticks, and subsequently determine which species is pathogenic or not. However, over the past 15 years, new data have contradicted this vision of rickettsiae [33]. As examples, R. parkeri, considered as non pathogenic for 65 years, was recently demonstrated to be a human pathogen [34]; R. canadensis is suspected epidemiologically and serologically to cause disease [35]; R. bellii has been shown to cause escharotic lesions when injected in guinea pigs [23] and may thus be pathogenic in humans. Finally, Coxiella burnetii (as R. diasporica), R. africae (as ESF agent), and Legionella pneumophila (as Tatlock agent) [36] were considered to be non-pathogenic rickettsiae in the past, before being recognized as human pathogens [33]. Recent findings demonstrated that the vector range of rickettsiae is not fully established [33]. For examples, R. prowazekii (a typical louse borne disease) was found in ticks in Africa and Mexico [37], [38] and was reported in lice and acarids from flying squirrels in the USA [39]. Similarly, R. conorii was reported to infect mites and lice [40], [41], and R. bellii was found in insects (unpublished data), although both species were believed to be strictly associated with ticks. Therefore, a simplification of the relationship between ecological niche, pathogenicity and phylotype is not possible, and these findings forced rickettsiologists to define more carefully rickettsiae as either pathogenic or of unknown pathogenicity. Moreover, by deliberately omitting SFG rickettsiae of unknown pathogenicity (such as the SFG species R. montanensis) the authors proposed in their Figure 1 a biased representation of what is currently known about rickettsial pathogenicity [19].
Discussion
We were surprised that the only hypothesis produced by Gillespie et al. [19] to explain the discordance between our results, showing the presence of two plasmids in R. felis [18], and those of Pornwiroon et al. who could detect only one plasmid [14], was that the small plasmid form of R. felis was an artefact of our genome assembly. It is clear in other bacterial genera that the plasmid content may vary from one strain to another and that plasmids may not be stably maintained in culture [42], [43]. In our previous work, we had shown the presence of the two plasmid forms [18], and demonstrated in the present article that R. felis may have one, two or no plasmid, depending on the strain or the culture passage history. This plasmidic instability, which we also identified in R. africae (unpublished data), was also described recently in R. peacockii [21]. This phenomenon poses the problem of the significance of the genomic sequences of rickettsiae passaged many times in cell culture prior to sequencing, such as R. prowazekii or R. conorii.
Regarding the phylogenetic position of R. felis, the choice of genes for infering phylogenies should be extremely careful. For example, Woese clearly demonstrated discrepancies between the phylogenies obtained using the ribosomal operon and amino-acyl –tRNA synthetases [44]. This is mainly due to the fact that bacteria have a core set of conserved and inherited genes, which may be used to establish phylogenies reflecting their true evolution, and a set of genes acquired by lateral gene transfer or recombination, which may provide biased phylogenies [45]. By selecting a set of specific genes, Gillespie et al. [19] proposed the creation of a specific phylogenetic position for R. felis, between SFG and TG rickettsiae, contradicting biological data and their own work [25]. Moreover, R. felis may be subject to gene recombination with R. typhi as these two rickettsiae can meet in the same host flea. However, the current position of R. felis and its clustering with R. australis and R. akari is exactly the same as that defined in as early as 1999 [46]. Herein, we performed a unique phylogenetic study of Rickettsia species based on the concatenation of their core gene set and demonstrated unambiguously that R. felis belongs to the spotted fever group. Finally, classification of rickettsiae based on current knowledge of host specificity is not reliable.
We also demonstrated for the first time using a cloning method in Vero cells that R. felis does not grow at 37°C. This result confirms the fact that authors who initially reported a culture of this species at 37°C [8]–[11] did not grow the current strain of R. felis [47]. Their data may have resulted from a contamination with R. typhi, which they later acknowledged [12]. This also explains why phenotypic traits initially described for R. felis were similar to those of R. typhi [8]–[11].
We believe that it was important to clarify the status of R. felis. We clearly demonstrated that R. felis is a SFG rickettsia, that it does not grow at 37°C and that it has, without any possible doubt, two plasmids. Finally, we think that it would have been fair, since our R. felis strain is available, to check the presence of plasmids prior concluding that our data resulted from an error.
Materials and Methods
PCR detection of pRF and pRFδ plasmids
R. felis strain URRWXCal2 T kept frozen at -80°C since initial isolation was cultivated in XTC2 cells as previously described [47]. DNA was extracted from freshly cultivated R. felis using the QIAmp Tissue kit (Qiagen, Hilden, Germany). The pRFδ plasmid was detected using the primers pRFa and pRFd (expected size 1,168 bp, Figure 1). The pRF plasmid was detected using the primer pairs pRFa-pRFb (expected size 159 bp) and pRFc-pRFd (expected size 1,342 bp, Figure 1b). We used two negative controls, i. e., sterile water and a PCR mix without DNA. The amplification conditions were as follows: 2.0 µL of DNA was mixed with 0.1 µL Platinum TaqDNA Pol High Fidelity polymerase (Invitrogen, Cergy, France), 2.5 µL High Fidelity PCR Buffer 10X, 0.5 µL of a 10mM dNTP mixture, 1 µL of 50 mM MgSO4, 0.5 µL of each primer (10mM), and 17.9 µL sterile water. Amplification conditions included an initial denaturation at 94°C for 2 min followed by 25 cycles comprised of 94°C for 30sec., 55°C for 30 sec., and 68°C for 2 min. PCR products were resolved in 1% agarose gels with ethidium bromide.
Plasmid detection kit
We prepared a kit that contained DNA from R. felis strain URRWXCal2 T (DNA1), R. conorii strain Malish 7 (DNA2) and R. africae strain ESF-5 (DNA3) (Table 3) extracted using the QIAmp Tissue kit (Qiagen), and PCR primers. All reagents in this kit were anonymized. Primers pRFa, pRFb, pRFc, and pRFd were renamed B, C, D, and E, respectively (Figure 1b). The kit was sent to five independent expert laboratories worldwide, including laboratories located in the USA (laboratory L1), Switzerland (L2), Greece (L3), Spain (L4), and Japan (L5). In addition to the above-described DNA samples, each kit contained the primer pairs B-E specific for the pRFδ plasmid, B-C and D-E specific for the pRF plasmid (Figure 1b), and AF (5′-CCTATGGCTATTATGCTTGC-3′)–AR (5′-ATTGCAAAAAGTACAGTGAACA-3′) specific for the citrate synthase (gltA)-encoding gene. Each primer pair was tested on each of the three DNA specimens (Table 1). For each PCR assay, two negative controls were used, i. e., sterile water and a PCR mix without DNA. For the B-E, B-C and D-E PCR assays, the amplification conditions were similar to those described above. For the AF-AR PCR assay, the amplification conditions were the following: 5.0 µL of DNA was mixed with 0.125 µL HotstarTaq Polymerase (Qiagen), 2.5 µL Buffer, 2.5 µL dNTP, 1 µL MgCl2, 0.5 µL of each primer, and 13.0 µL sterile water. Amplification conditions included an initial denaturation at 94°C for 15 min followed by 39 cycles comprised of 94°C for 1 min., 54°C for 30 sec., and 72°C for 2 min. The reaction was completed by a final elongation step at 72°C for 5 min.
Table 3. DNA specimens sent to expert laboratories.
| Anonymized DNA label | Species | Strain |
| DNA1 | R. felis | URRWXCal2 T (ATCC VR1525) |
| DNA2 | R. conorii | Malish 7 T (ATCC VR613) |
| DNA3 | R. africae | ESF-5T |
Variation of plasmid content in R. felis
K. Macaluso provided the expert laboratory L1 with DNA from five R. felis-positive (strain LSU) C. felis fleas [14]. This DNA was tested using the four above-described primer pairs and PCR conditions. We also tested 64 Archaeopsylla erinacei fleas from Algeria previously found to contain R. felis strain Rf2125 [22].
Detection of pRF and pRFδ plasmids in single R. felis cells
Using tenfold dilutions of a suspension containing 104 plaque forming units of R. felis strain URRWXCal2 T (40 cell culture passages since initial isolation), we inoculated Vero cells at 30°C and 37°C and performed a plaque assay as previously described [48]. Then, we collected individually 20 R. felis clones grown from single R. felis cells (Figure 2). DNA extraction from each clone, and plasmid detection, were performed as described above. As positive control, we used DNA extracted from our current R. felis strain URRWXCal2 T culture (50 cell culture passages).
Phylogenetic analysis
To estimate the phylogenetic position of R. felis among Rickettsia species, we based our analysis on the 704 core protein-coding genes identified by Blanc et al. by comparison of 7 rickettsial genomes [32]. Of these genes, a total of 667 complete orthologous genes were found using the Blast software in the R. slovaca and R. akari genomes [49]. Subsequently, the amino acid sequences of these 667 proteins were concatenated for each genome and multiple alignment was performed using the Mafft software [50]. Gapped positions were removed. The maximum parsimony and neighbor joining trees were constructed using the MEGA 3.1 software [51]. Branching support was evaluated using the bootstrap method.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to declare.
References
- 1.Adams JR, Schmidtmann ET, Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouché), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–409. doi: 10.4269/ajtmh.1990.43.400. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, La Scola B, Enea M, Fournier PE, Roux V, et al. A flea-associated Rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7:73–81. doi: 10.3201/eid0701.010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–1080. doi: 10.1016/S0140-6736(00)02735-5. [DOI] [PubMed] [Google Scholar]
- 4.Schriefer ME, Sacci JB, Jr, Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32:949–954. doi: 10.1128/jcm.32.4.949-954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter J, Fournier PE, Petridou J, Häussinger D, Raoult D. Rickettsia felis infection acquired in Europe and documented by Polymerase Chain Reaction. Emerg Infect Dis. 2002;8:207–208. doi: 10.3201/eid0802.010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parola P, Miller RS, McDaniel P, Telford SR, III, Rolain JM, et al. Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis. 2003;9:592–595. doi: 10.3201/eid0905.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azad AF, Sacci JB, Nelson WM, Dasch GA, Schmidtmann ET, et al. Genetic characterization and transovarial transmission of a typhus-like rickettsia found in cat fleas. Proc Natl Acad Sci USA. 1992;89:43–46. doi: 10.1073/pnas.89.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JA, Radulovic S, Schriefer ME, Azad AF. Rickettsia felis : a new species of pathogenic rickettsia isolated from cat fleas. J Clin Microbiol. 1996;34:671–674. doi: 10.1128/jcm.34.3.671-674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radulovic S, Higgins JA, Jaworski DC, Dasch GA, Azad AF. Isolation, cultivation, and partial characterization of the ELB agent associated with cat fleas. Infect Immun. 1995;63:4826–4829. doi: 10.1128/iai.63.12.4826-4829.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radulovic S, Higgins JA, Jaworski DC, Azad AF. In vitro and in vivo antibiotic susceptibilities of ELB rickettsiae. Antimicrob Agents Chemother. 1995;39:2564–2566. doi: 10.1128/aac.39.11.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne rickettsioses : ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radulovic S, Higgins JA, Jaworski DC, Azad AF. Erratum to “In vitro and in vivo antibiotic susceptibilities of ELB rickettsiae”. Antimicrob Agents Chemother. 1996;40:2912. doi: 10.1128/aac.39.11.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noden BH, Radulovic S, Higgins JA, Azad AF. Molecular identification of Rickettsia typhi and R.felis in co-infected Ctenocephalides felis (Siphonaptera: Pulicidae). J Med Entomol. 1998;35:410–414. doi: 10.1093/jmedent/35.4.410. [DOI] [PubMed] [Google Scholar]
- 14.Pornwiroon W, Pourciau SS, Foil LD, Macaluso KR. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl Environ Microbiol. 2006;72:5589–5595. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horta MC, Labruna MB, Durigon EL, Schumaker TT. Isolation of Rickettsia felis in the mosquito cell line C6/36. Appl Environ Microbiol. 2006;72:1705–1707. doi: 10.1128/AEM.72.2.1705-1707.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolain JM, Sthul L, Maurin M, Raoult D. Evaluation of antibiotic susceptibilities of three Rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob Agents Chemother. 2002;46:2747–2751. doi: 10.1128/AAC.46.9.2747-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang R, Raoult D. Antigenic classification of Rickettsia felis by using monoclonal and polyclonal antibodies. Clin Diagn Lab Immunol. 2003;10:221–228. doi: 10.1128/CDLI.10.2.221-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. The Genome Sequence of Rickettsia felis Identifies the First Putative Conjugative Plasmid in an Obligate Intracellular Parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, et al. Plasmids and Rickettsial Evolution: Insight from Rickettsia felis. PLoS ONE. 2007;2:e266. doi: 10.1371/journal.pone.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson G, Foil LD. Efficacy of diflubenzuron in simulated household and yard conditions against the cat flea Ctenocephalides felis (Bouche) (Siphonoptera: Pulicidae). J Med Entomol. 1993;30:619–621. doi: 10.1093/jmedent/30.3.619. [DOI] [PubMed] [Google Scholar]
- 21.Baldridge GD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG. Plasmids of the pRM/pRF Family Occur in Diverse Rickettsia species. Appl Environ Microbiol. 2007 doi: 10.1128/AEM.02262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitam I, Parola P, De La Cruz KD, Matsumoto K, Baziz B, et al. First molecular detection of Rickettsia felis in fleas from Algeria. Am J Trop Med Hyg. 2006;74:532–535. [PubMed] [Google Scholar]
- 23.Ogata H, La Scola B, Audic S, Renesto P, Blanc G, et al. Genome Sequence of Rickettsia bellii Illuminates the Role of Amoebae in Gene Exchanges between Intracellular Pathogens. PLoS Genet. 2006;2:e76. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abergel C, Blanc G, Monchois V, Renesto P, Sigoillot C, et al. Impact of the excision of an ancient repeat insertion on Rickettsia conorii guanylate kinase activity. Mol Biol Evol. 2006;23:2112–2122. doi: 10.1093/molbev/msl082. [DOI] [PubMed] [Google Scholar]
- 25.Bouyer DH, Stenos J, Crocquet-Valdes P, Moron C, Vsevolod P, et al. Rickettsia felis : molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. 2001;51:339–347. doi: 10.1099/00207713-51-2-339. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JA, Sacci JB, Schriefer ME, Endris RG, Azad AF. Molecular identification of rickettsia-like microorganisms associated with colonized cat fleas (Ctenocephalides felis). Insect Molecular Biology. 1994;3:27–33. doi: 10.1111/j.1365-2583.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 27.Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol. 2001;51:1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]
- 28.Ngwamidiba M, Blanc G, Raoult D, Fournier PE. Sca1, a previously undescribed paralog from autotransporter protein-encoding genes in Rickettsiaspecies. BMC Microbiol. 2006;6:12. doi: 10.1186/1471-2180-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier PE, Raoult D. Bacteriology, taxonomy, and phylogeny of Rickettsia. In: Raoult D, Parola P, editors. Rickettsial diseases. New York: Informa healthcare; 2007. pp. 1–13. [Google Scholar]
- 30.Stenos J, Walker DH. The rickettsial outer-membrane protein A and B genes of Rickettsia australis divergent rickettsia of the spotted fever group. Int J Syst Evol Microbiol. 2000;50:1775–1779. doi: 10.1099/00207713-50-5-1775. [DOI] [PubMed] [Google Scholar]
- 31.Wolf YI, Aravind L, Koonin EV. Rickettsiae and Chlamydiae evidence of horizontal gene tranfer and gene exchange. Trends Genet. 1999;15:173–175. doi: 10.1016/s0168-9525(99)01704-7. [DOI] [PubMed] [Google Scholar]
- 32.Blanc G, Ogata H, Robert C, Audic S, Suhre K, et al. Reductive genome evolution from the mother of Rickettsia. PLOS Genet. 2007;3:e14. doi: 10.1371/journal.pgen.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;15:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 35.Bozeman FM, Elisberg BL, Humphries JW, Runcik K, Palmer DB., Jr Serologic evidence of Rickettsia canada infection of man. J Infect Dis. 1970;121:367–371. doi: 10.1093/infdis/121.4.367. [DOI] [PubMed] [Google Scholar]
- 36.Hebert GA, Moss CW, McDougal LK, Bozeman FM, McKinney RM, et al. The rickettsia-like organisms TATLOCK (1943) and HEBA (1959): bacteria phenotypically similar to but genetically distinct from Legionella pneumophila and the WIGA bacterium. Ann Med Inter. 1980;92:45–52. doi: 10.7326/0003-4819-92-1-45. [DOI] [PubMed] [Google Scholar]
- 37.Medina-Sanchez A, Bouyer DH, cantara-Rodriguez V, Mafra C, Zavala-Castro J, et al. Detection of a typhus group Rickettsia in Amblyomma ticks in the state of Nuevo Leon, Mexico. Ann N Y Acad Sci. 2005;1063:327–332. doi: 10.1196/annals.1355.052. [DOI] [PubMed] [Google Scholar]
- 38.Reiss-Gutfreund RJ. The isolation of Rickettsia prowazeki and mooseri from unusual sources. Am J Trop Med Hyg. 1966;15:943–949. doi: 10.4269/ajtmh.1966.15.943. [DOI] [PubMed] [Google Scholar]
- 39.Bozeman FM, Masiello SA, Williams MS, Elisberg BL. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975;255:545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- 40.Houhamdi L, Raoult D. Experimentally infected human body lice (Pediculus humanus humanus) as vectors of Rickettsia rickettsii and Rickettsia conorii in a rabbit model. Am J Trop Med Hyg. 2006;74:521–525. [PubMed] [Google Scholar]
- 41.Choi YJ, Lee EM, Park JM, Lee KM, Han SH, et al. Molecular detection of various rickettsiae in mites (acari: trombiculidae) in southern Jeolla Province, Korea. Microbiol Immunol. 2007;51:307–312. doi: 10.1111/j.1348-0421.2007.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 42.Welch RA, Burland V, Plunkett G, III, Redford P, Roesch P, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng W, Burland V, Plunkett G, III, Boutin A, Mayhew GF, et al. Genome sequence of Yersinia pestis KIM. J Bacteriol. 2002;184:4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woese CR, Olsen GJ, Ibba M, Söll D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol Mol Biol Rev. 2000;64:202–236. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux V. Phylogenetic analysis and taxonomic relationships among the genus Rickettsia. In: Raoult D, Brouqui P, editors. Rickettsiae and Rickettsial diseases at the turn of the third millinium. Marseille: Elsevier; 1999. pp. 52–66. [Google Scholar]
- 47.La Scola B, Meconi S, Fenollar F, Rolain JM, Roux V, et al. Emended description of Rickettsia felis (Bouyer et al. 2001) a temperature-dependant cultured bacterium. Int J Syst Evol Microbiol. 2002;52:2035–2041. doi: 10.1099/00207713-52-6-2035. [DOI] [PubMed] [Google Scholar]
- 48.Rolain JM, Maurin M, Vestris G, Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998;42:1537–1541. doi: 10.1128/aac.42.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]